 Found 375 hits with Last Name = 'durden' and Initial = 'dl'
Found 375 hits with Last Name = 'durden' and Initial = 'dl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bromodomain-containing protein 4 [368-440]
(Homo sapiens (Human)) | BDBM259884

(US10308662, Compound 125 | US9505780, 125)Show SMILES CCOC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCSCC1 Show InChI InChI=1S/C20H19NO4S2/c1-2-24-20(23)14-5-3-13(4-6-14)15-12-27-19-16(22)11-17(25-18(15)19)21-7-9-26-10-8-21/h3-6,11-12H,2,7-10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
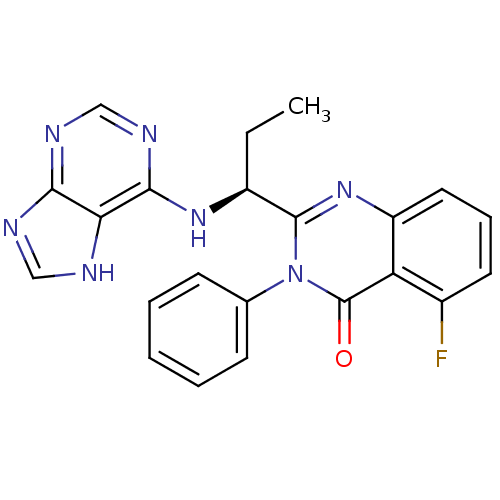
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427456

(CHEMBL2326953 | US10308662, Compound 44 | US950578...)Show SMILES COC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C19H17NO5S/c1-23-19(22)13-4-2-12(3-5-13)14-11-26-18-15(21)10-16(25-17(14)18)20-6-8-24-9-7-20/h2-5,10-11H,6-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427456

(CHEMBL2326953 | US10308662, Compound 44 | US950578...)Show SMILES COC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C19H17NO5S/c1-23-19(22)13-4-2-12(3-5-13)14-11-26-18-15(21)10-16(25-17(14)18)20-6-8-24-9-7-20/h2-5,10-11H,6-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427456

(CHEMBL2326953 | US10308662, Compound 44 | US950578...)Show SMILES COC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C19H17NO5S/c1-23-19(22)13-4-2-12(3-5-13)14-11-26-18-15(21)10-16(25-17(14)18)20-6-8-24-9-7-20/h2-5,10-11H,6-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427485

(CHEMBL2326959 | US10308662, Compound 120 | US95057...)Show SMILES O=C(Nc1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1)c1ccncc1 Show InChI InChI=1S/C23H19N3O4S/c27-19-13-20(26-9-11-29-12-10-26)30-21-18(14-31-22(19)21)15-1-3-17(4-2-15)25-23(28)16-5-7-24-8-6-16/h1-8,13-14H,9-12H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427455
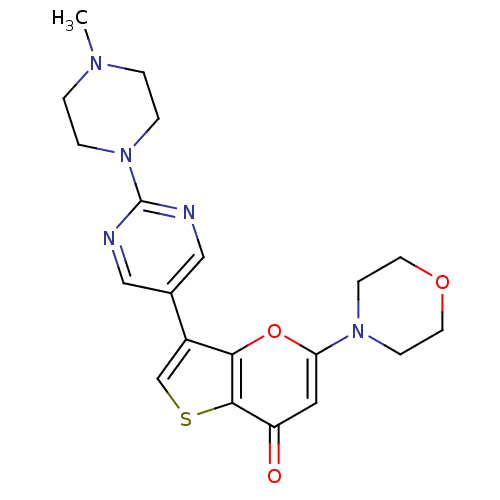
(CHEMBL2326951)Show SMILES CN1CCN(CC1)c1ncc(cn1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C20H23N5O3S/c1-23-2-4-25(5-3-23)20-21-11-14(12-22-20)15-13-29-19-16(26)10-17(28-18(15)19)24-6-8-27-9-7-24/h10-13H,2-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427489
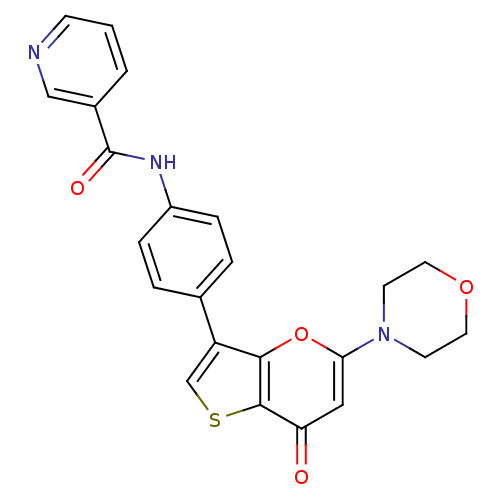
(CHEMBL2326955)Show SMILES O=C(Nc1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1)c1cccnc1 Show InChI InChI=1S/C23H19N3O4S/c27-19-12-20(26-8-10-29-11-9-26)30-21-18(14-31-22(19)21)15-3-5-17(6-4-15)25-23(28)16-2-1-7-24-13-16/h1-7,12-14H,8-11H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427485

(CHEMBL2326959 | US10308662, Compound 120 | US95057...)Show SMILES O=C(Nc1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1)c1ccncc1 Show InChI InChI=1S/C23H19N3O4S/c27-19-13-20(26-9-11-29-12-10-26)30-21-18(14-31-22(19)21)15-1-3-17(4-2-15)25-23(28)16-5-7-24-8-6-16/h1-8,13-14H,9-12H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427485

(CHEMBL2326959 | US10308662, Compound 120 | US95057...)Show SMILES O=C(Nc1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1)c1ccncc1 Show InChI InChI=1S/C23H19N3O4S/c27-19-13-20(26-9-11-29-12-10-26)30-21-18(14-31-22(19)21)15-1-3-17(4-2-15)25-23(28)16-5-7-24-8-6-16/h1-8,13-14H,9-12H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427459
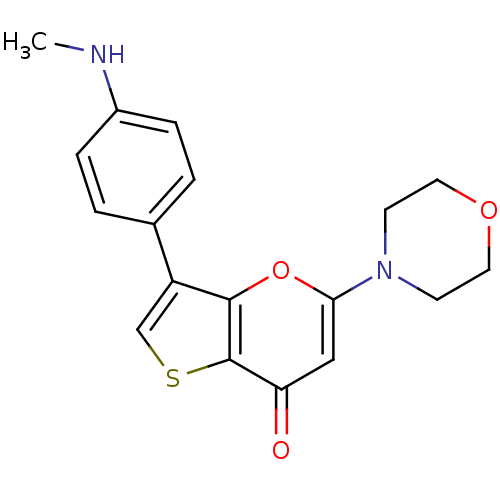
(CHEMBL2326954)Show InChI InChI=1S/C18H18N2O3S/c1-19-13-4-2-12(3-5-13)14-11-24-18-15(21)10-16(23-17(14)18)20-6-8-22-9-7-20/h2-5,10-11,19H,6-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427457

(CHEMBL2326952 | US10308662, Compound 25 | US950578...)Show SMILES CCOC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C20H19NO5S/c1-2-25-20(23)14-5-3-13(4-6-14)15-12-27-19-16(22)11-17(26-18(15)19)21-7-9-24-10-8-21/h3-6,11-12H,2,7-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427457

(CHEMBL2326952 | US10308662, Compound 25 | US950578...)Show SMILES CCOC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C20H19NO5S/c1-2-25-20(23)14-5-3-13(4-6-14)15-12-27-19-16(22)11-17(26-18(15)19)21-7-9-24-10-8-21/h3-6,11-12H,2,7-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427457

(CHEMBL2326952 | US10308662, Compound 25 | US950578...)Show SMILES CCOC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C20H19NO5S/c1-2-25-20(23)14-5-3-13(4-6-14)15-12-27-19-16(22)11-17(26-18(15)19)21-7-9-24-10-8-21/h3-6,11-12H,2,7-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [75-147]
(Homo sapiens (Human)) | BDBM259897

(US9505780, JQ-1)Show SMILES Cc1nnc2[C@@H](N=C(c3c(C)c(C)sc3-n12)c1ccc(Cl)cc1)C(=O)OC(C)(C)C |c:6| Show InChI InChI=1S/C22H23ClN4O2S/c1-11-12(2)30-20-16(11)17(14-7-9-15(23)10-8-14)24-18(21(28)29-22(4,5)6)19-26-25-13(3)27(19)20/h7-10,18H,1-6H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 27.1 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [44-167]
(Homo sapiens (Human)) | BDBM50365262

((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...)Show SMILES Cc1nnc2[C@H](CC(=O)OC(C)(C)C)N=C(c3c(C)c(C)sc3-n12)c1ccc(Cl)cc1 |r,c:14| Show InChI InChI=1S/C23H25ClN4O2S/c1-12-13(2)31-22-19(12)20(15-7-9-16(24)10-8-15)25-17(11-18(29)30-23(4,5)6)21-27-26-14(3)28(21)22/h7-10,17H,11H2,1-6H3/t17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 27.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427453
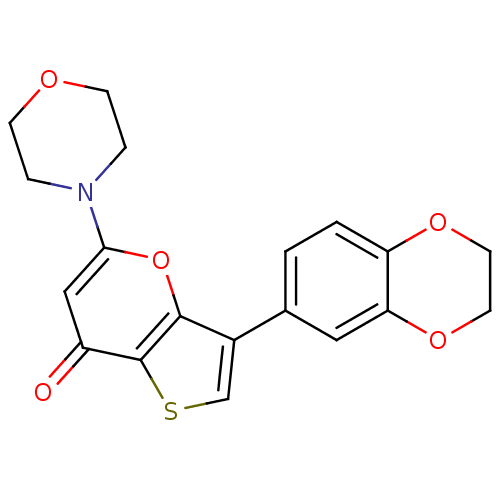
(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427453

(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha
(Homo sapiens (Human)) | BDBM50427453

(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [368-440]
(Homo sapiens (Human)) | BDBM259897

(US9505780, JQ-1)Show SMILES Cc1nnc2[C@@H](N=C(c3c(C)c(C)sc3-n12)c1ccc(Cl)cc1)C(=O)OC(C)(C)C |c:6| Show InChI InChI=1S/C22H23ClN4O2S/c1-11-12(2)30-20-16(11)17(14-7-9-15(23)10-8-14)24-18(21(28)29-22(4,5)6)19-26-25-13(3)27(19)20/h7-10,18H,1-6H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 39.1 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [349-460]
(Homo sapiens (Human)) | BDBM50365262

((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...)Show SMILES Cc1nnc2[C@H](CC(=O)OC(C)(C)C)N=C(c3c(C)c(C)sc3-n12)c1ccc(Cl)cc1 |r,c:14| Show InChI InChI=1S/C23H25ClN4O2S/c1-12-13(2)31-22-19(12)20(15-7-9-16(24)10-8-15)25-17(11-18(29)30-23(4,5)6)21-27-26-14(3)28(21)22/h7-10,17H,11H2,1-6H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 39.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50427453

(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427489

(CHEMBL2326955)Show SMILES O=C(Nc1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1)c1cccnc1 Show InChI InChI=1S/C23H19N3O4S/c27-19-12-20(26-8-10-29-11-9-26)30-21-18(14-31-22(19)21)15-3-5-17(6-4-15)25-23(28)16-2-1-7-24-13-16/h1-7,12-14H,8-11H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM259879
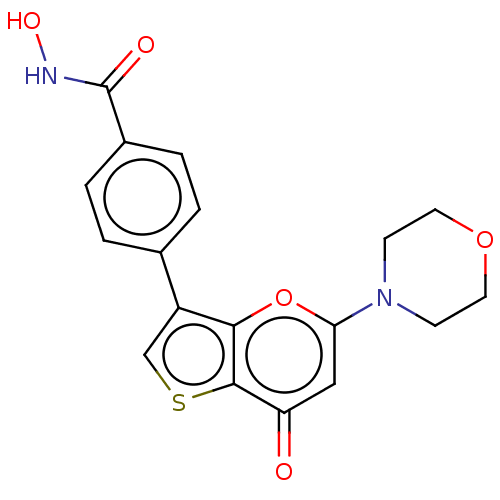
(US10308662, Compound 118 | US9505780, 118)Show SMILES ONC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C18H16N2O5S/c21-14-9-15(20-5-7-24-8-6-20)25-16-13(10-26-17(14)16)11-1-3-12(4-2-11)18(22)19-23/h1-4,9-10,23H,5-8H2,(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM259879

(US10308662, Compound 118 | US9505780, 118)Show SMILES ONC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C18H16N2O5S/c21-14-9-15(20-5-7-24-8-6-20)25-16-13(10-26-17(14)16)11-1-3-12(4-2-11)18(22)19-23/h1-4,9-10,23H,5-8H2,(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha
(Homo sapiens (Human)) | BDBM50427488

(CHEMBL2326956 | US10308662, Compound 100 | US95057...)Show InChI InChI=1S/C18H15NO5S/c20-14-9-15(19-5-7-23-8-6-19)24-16-13(10-25-17(14)16)11-1-3-12(4-2-11)18(21)22/h1-4,9-10H,5-8H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 55.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427488

(CHEMBL2326956 | US10308662, Compound 100 | US95057...)Show InChI InChI=1S/C18H15NO5S/c20-14-9-15(19-5-7-23-8-6-19)24-16-13(10-25-17(14)16)11-1-3-12(4-2-11)18(21)22/h1-4,9-10H,5-8H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 55.2 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427459

(CHEMBL2326954)Show InChI InChI=1S/C18H18N2O3S/c1-19-13-4-2-12(3-5-13)14-11-24-18-15(21)10-16(23-17(14)18)20-6-8-22-9-7-20/h2-5,10-11,19H,6-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427492

(CHEMBL2322244)Show InChI InChI=1S/C18H17NO4S/c1-21-13-4-2-12(3-5-13)14-11-24-18-15(20)10-16(23-17(14)18)19-6-8-22-9-7-19/h2-5,10-11H,6-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427480
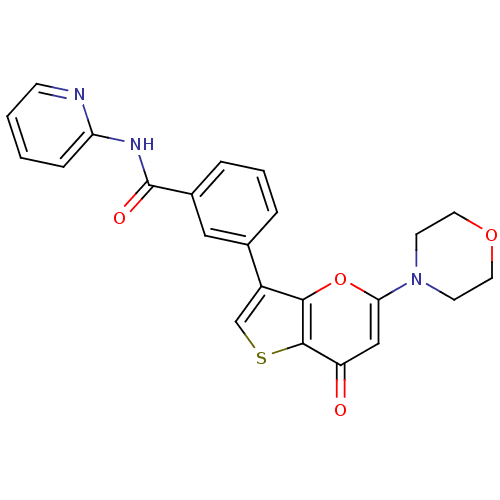
(CHEMBL2326964)Show SMILES O=C(Nc1ccccn1)c1cccc(c1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C23H19N3O4S/c27-18-13-20(26-8-10-29-11-9-26)30-21-17(14-31-22(18)21)15-4-3-5-16(12-15)23(28)25-19-6-1-2-7-24-19/h1-7,12-14H,8-11H2,(H,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427481
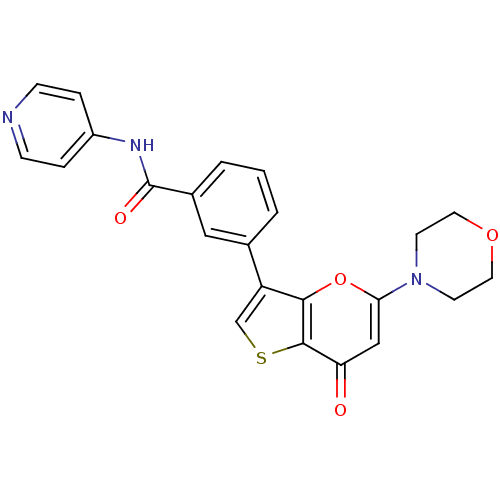
(CHEMBL2326963)Show SMILES O=C(Nc1ccncc1)c1cccc(c1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C23H19N3O4S/c27-19-13-20(26-8-10-29-11-9-26)30-21-18(14-31-22(19)21)15-2-1-3-16(12-15)23(28)25-17-4-6-24-7-5-17/h1-7,12-14H,8-11H2,(H,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427467
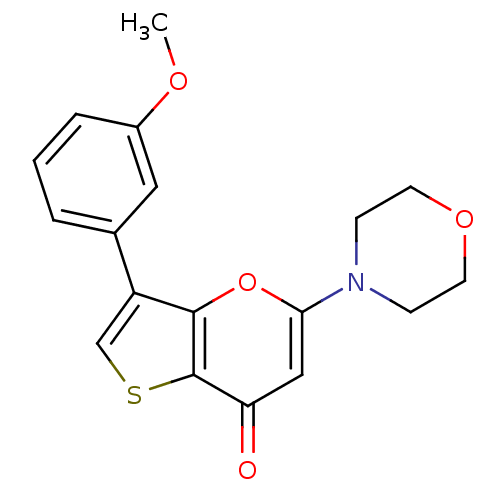
(CHEMBL2322229)Show InChI InChI=1S/C18H17NO4S/c1-21-13-4-2-3-12(9-13)14-11-24-18-15(20)10-16(23-17(14)18)19-5-7-22-8-6-19/h2-4,9-11H,5-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427493
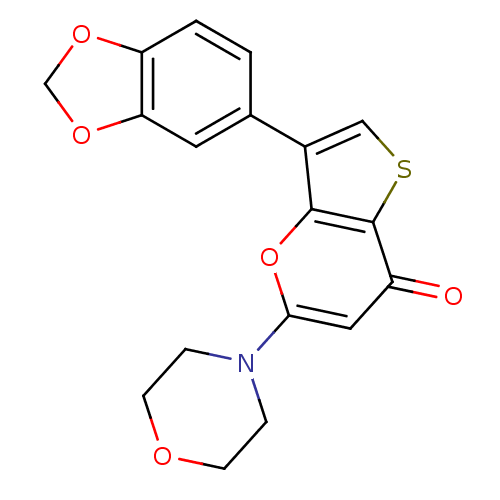
(CHEMBL2322243)Show InChI InChI=1S/C18H15NO5S/c20-13-8-16(19-3-5-21-6-4-19)24-17-12(9-25-18(13)17)11-1-2-14-15(7-11)23-10-22-14/h1-2,7-9H,3-6,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427475

(CHEMBL2326967)Show SMILES O=c1cc(oc2c(csc12)-c1cc2ccccc2[nH]1)N1CCOCC1 Show InChI InChI=1S/C19H16N2O3S/c22-16-10-17(21-5-7-23-8-6-21)24-18-13(11-25-19(16)18)15-9-12-3-1-2-4-14(12)20-15/h1-4,9-11,20H,5-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427488

(CHEMBL2326956 | US10308662, Compound 100 | US95057...)Show InChI InChI=1S/C18H15NO5S/c20-14-9-15(19-5-7-23-8-6-19)24-16-13(10-25-17(14)16)11-1-3-12(4-2-11)18(21)22/h1-4,9-10H,5-8H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427460
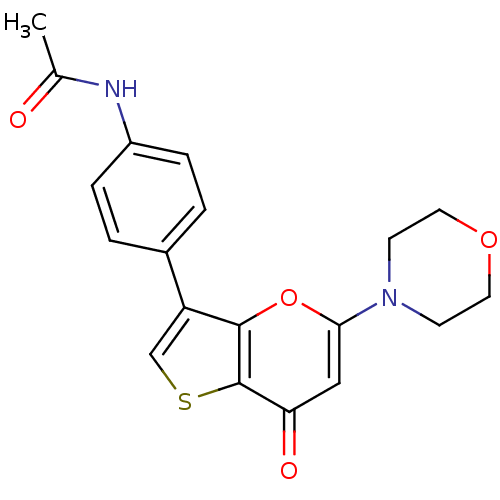
(CHEMBL2322236)Show SMILES CC(=O)Nc1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C19H18N2O4S/c1-12(22)20-14-4-2-13(3-5-14)15-11-26-19-16(23)10-17(25-18(15)19)21-6-8-24-9-7-21/h2-5,10-11H,6-9H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427482
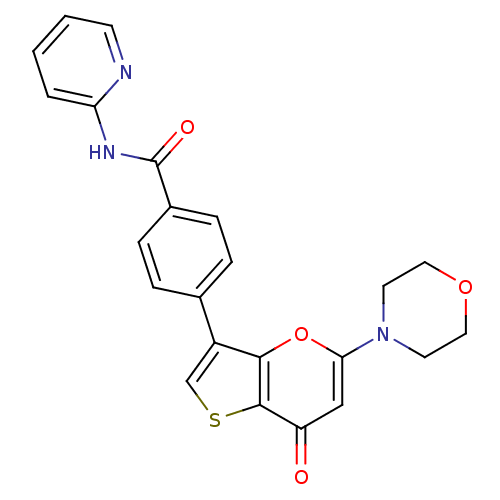
(CHEMBL2326962)Show SMILES O=C(Nc1ccccn1)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C23H19N3O4S/c27-18-13-20(26-9-11-29-12-10-26)30-21-17(14-31-22(18)21)15-4-6-16(7-5-15)23(28)25-19-3-1-2-8-24-19/h1-8,13-14H,9-12H2,(H,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50403068

(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110gamma (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha
(Homo sapiens (Human)) | BDBM259879

(US10308662, Compound 118 | US9505780, 118)Show SMILES ONC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C18H16N2O5S/c21-14-9-15(20-5-7-24-8-6-20)25-16-13(10-26-17(14)16)11-1-3-12(4-2-11)18(22)19-23/h1-4,9-10,23H,5-8H2,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM259879

(US10308662, Compound 118 | US9505780, 118)Show SMILES ONC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C18H16N2O5S/c21-14-9-15(20-5-7-24-8-6-20)25-16-13(10-26-17(14)16)11-1-3-12(4-2-11)18(22)19-23/h1-4,9-10,23H,5-8H2,(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha
(Homo sapiens (Human)) | BDBM50427485

(CHEMBL2326959 | US10308662, Compound 120 | US95057...)Show SMILES O=C(Nc1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1)c1ccncc1 Show InChI InChI=1S/C23H19N3O4S/c27-19-13-20(26-9-11-29-12-10-26)30-21-18(14-31-22(19)21)15-1-3-17(4-2-15)25-23(28)16-5-7-24-8-6-16/h1-8,13-14H,9-12H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427491
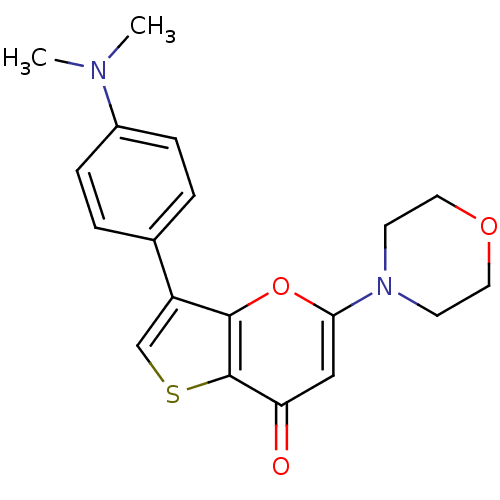
(CHEMBL2322245)Show InChI InChI=1S/C19H20N2O3S/c1-20(2)14-5-3-13(4-6-14)15-12-25-19-16(22)11-17(24-18(15)19)21-7-9-23-10-8-21/h3-6,11-12H,7-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427485

(CHEMBL2326959 | US10308662, Compound 120 | US95057...)Show SMILES O=C(Nc1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1)c1ccncc1 Show InChI InChI=1S/C23H19N3O4S/c27-19-13-20(26-9-11-29-12-10-26)30-21-18(14-31-22(19)21)15-1-3-17(4-2-15)25-23(28)16-5-7-24-8-6-16/h1-8,13-14H,9-12H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427463
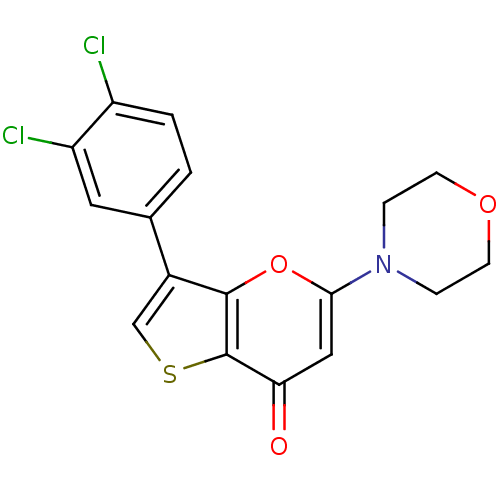
(CHEMBL2322233)Show InChI InChI=1S/C17H13Cl2NO3S/c18-12-2-1-10(7-13(12)19)11-9-24-17-14(21)8-15(23-16(11)17)20-3-5-22-6-4-20/h1-2,7-9H,3-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427484
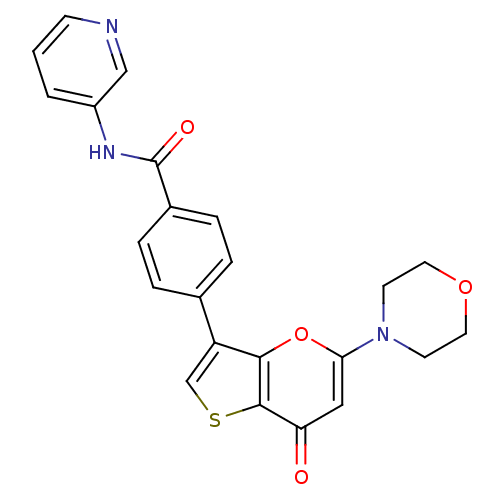
(CHEMBL2326960)Show SMILES O=C(Nc1cccnc1)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C23H19N3O4S/c27-19-12-20(26-8-10-29-11-9-26)30-21-18(14-31-22(19)21)15-3-5-16(6-4-15)23(28)25-17-2-1-7-24-13-17/h1-7,12-14H,8-11H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427469
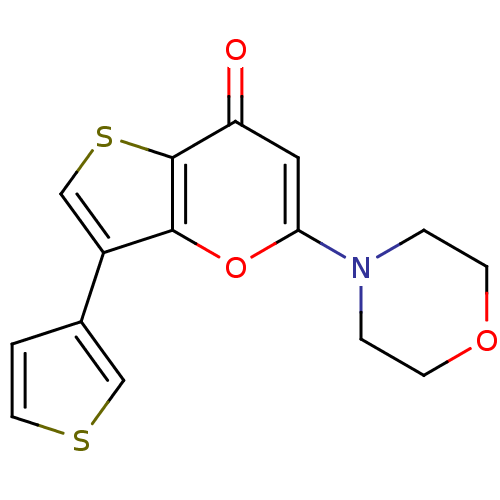
(CHEMBL2322226)Show InChI InChI=1S/C15H13NO3S2/c17-12-7-13(16-2-4-18-5-3-16)19-14-11(9-21-15(12)14)10-1-6-20-8-10/h1,6-9H,2-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427464

(CHEMBL2322232)Show InChI InChI=1S/C17H14ClNO3S/c18-12-3-1-11(2-4-12)13-10-23-17-14(20)9-15(22-16(13)17)19-5-7-21-8-6-19/h1-4,9-10H,5-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427471
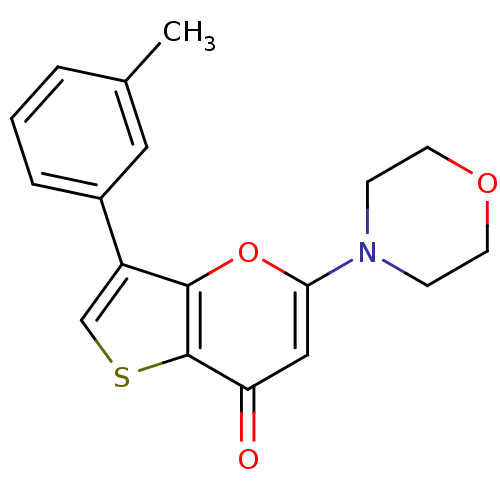
(CHEMBL2326971)Show InChI InChI=1S/C18H17NO3S/c1-12-3-2-4-13(9-12)14-11-23-18-15(20)10-16(22-17(14)18)19-5-7-21-8-6-19/h2-4,9-11H,5-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427460

(CHEMBL2322236)Show SMILES CC(=O)Nc1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C19H18N2O4S/c1-12(22)20-14-4-2-13(3-5-14)15-11-26-19-16(23)10-17(25-18(15)19)21-6-8-24-9-7-21/h2-5,10-11H,6-9H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50427477
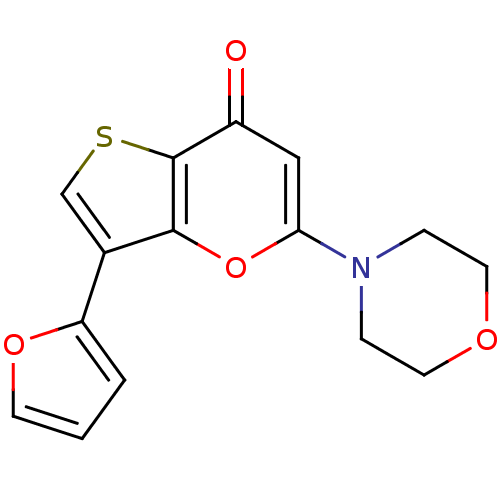
(CHEMBL2322242)Show InChI InChI=1S/C15H13NO4S/c17-11-8-13(16-3-6-18-7-4-16)20-14-10(9-21-15(11)14)12-2-1-5-19-12/h1-2,5,8-9H,3-4,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data