 Found 115 hits with Last Name = 'el-gamal' and Initial = 'r'
Found 115 hits with Last Name = 'el-gamal' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM13058
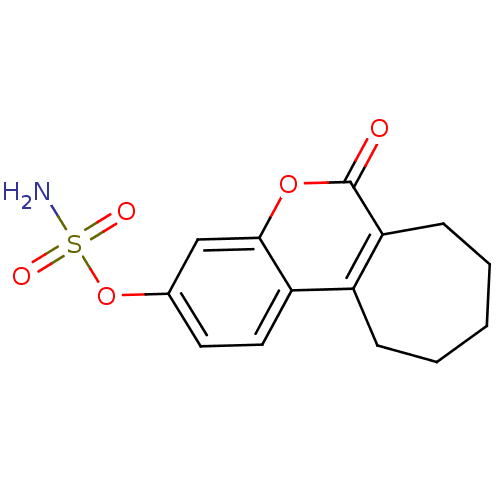
(6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...)Show InChI InChI=1S/C14H15NO5S/c15-21(17,18)20-9-6-7-11-10-4-2-1-3-5-12(10)14(16)19-13(11)8-9/h6-8H,1-5H2,(H2,15,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate incubated for 20 hrs by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BOMCC as a substrate incubated for 20 mins by fluorescence based analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50574707
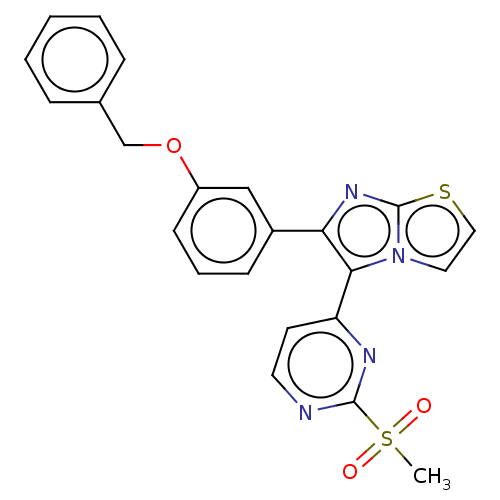
(CHEMBL4860202)Show SMILES CS(=O)(=O)c1nccc(n1)-c1c(nc2sccn12)-c1cccc(OCc2ccccc2)c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ErbB4 in human T47D cells assessed as suppression of neuregulin 1-induced autophosphorylation incubated for 90 mins by sandwich ELISA |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM13058

(6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...)Show InChI InChI=1S/C14H15NO5S/c15-21(17,18)20-9-6-7-11-10-4-2-1-3-5-12(10)14(16)19-13(11)8-9/h6-8H,1-5H2,(H2,15,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell lysates using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114434
BindingDB Entry DOI: 10.7270/Q2833X0F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK3 (unknown origin) incubated for 40 mins in presence of Mg/ATP mix by [gamma p33]-ATP based scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) incubated for 40 mins in presence of Mg/ATP mix by [gamma p33]-ATP based scintillation counting meth... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50541451
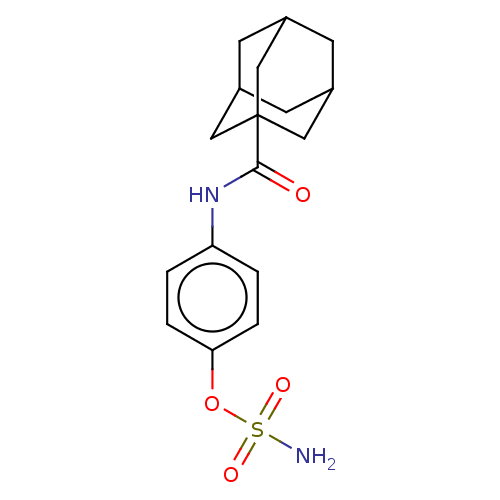
(CHEMBL4635151)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)C23CC4CC(CC(C4)C2)C3)cc1 |TLB:10:12:15:19.17.18,THB:17:16:13:19.18.20,17:18:15.16.21:13,20:18:15:21.12.13,20:12:15:19.17.18| Show InChI InChI=1S/C17H22N2O4S/c18-24(21,22)23-15-3-1-14(2-4-15)19-16(20)17-8-11-5-12(9-17)7-13(6-11)10-17/h1-4,11-13H,5-10H2,(H,19,20)(H2,18,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate incubated for 20 hrs by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50574707

(CHEMBL4860202)Show SMILES CS(=O)(=O)c1nccc(n1)-c1c(nc2sccn12)-c1cccc(OCc2ccccc2)c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ErbB4 (unknown origin) incubated for 40 mins in presence of Mg/ATP mix by [gamma p33]-ATP based scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50574708
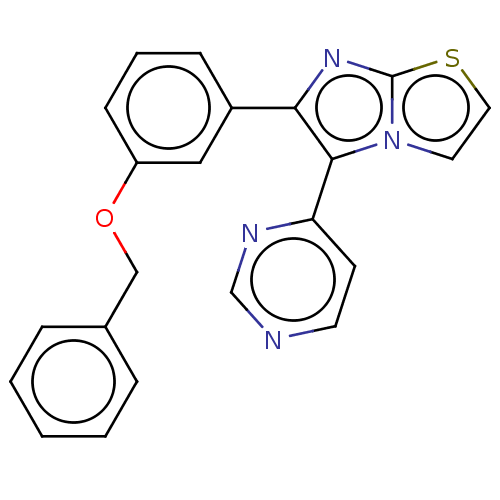
(CHEMBL4866102)Show SMILES C(Oc1cccc(c1)-c1nc2sccn2c1-c1ccncn1)c1ccccc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ErbB4 (unknown origin) incubated for 40 mins in presence of Mg/ATP mix by [gamma p33]-ATP based scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50117930
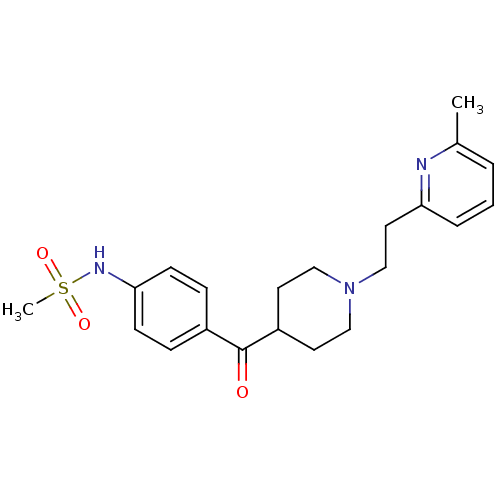
((4-{1-[2-(6-Methyl-pyridin-2-yl)-ethyl]-piperidine...)Show SMILES Cc1cccc(CCN2CCC(CC2)C(=O)c2ccc(NS(C)(=O)=O)cc2)n1 Show InChI InChI=1S/C21H27N3O3S/c1-16-4-3-5-19(22-16)12-15-24-13-10-18(11-14-24)21(25)17-6-8-20(9-7-17)23-28(2,26)27/h3-9,18,23H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG incubated for 3 hrs by competitive fluorescence tracer binding based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50541453
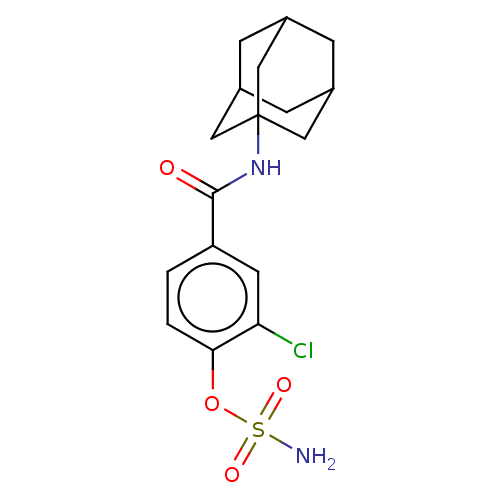
(CHEMBL4637433)Show SMILES NS(=O)(=O)Oc1ccc(cc1Cl)C(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:14:15:18:22.20.21,THB:20:19:16:22.21.23,20:21:18.19.24:16,23:21:18:24.15.16,23:15:18:22.20.21| Show InChI InChI=1S/C17H21ClN2O4S/c18-14-6-13(1-2-15(14)24-25(19,22)23)16(21)20-17-7-10-3-11(8-17)5-12(4-10)9-17/h1-2,6,10-12H,3-5,7-9H2,(H,20,21)(H2,19,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell lysates using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Flt4 (unknown origin) in presence of [gamma33]-ATP by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113081
BindingDB Entry DOI: 10.7270/Q2B85CV4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) incubated for 40 mins in presence of Mg/ATP mix by [gamma p33]-ATP based scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50591872
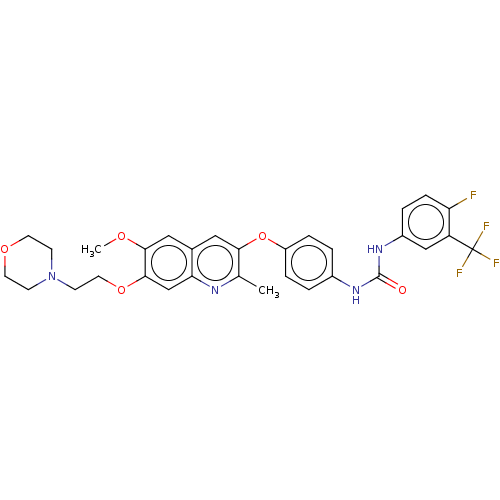
(CHEMBL5202188)Show SMILES COc1cc2cc(Oc3ccc(NC(=O)Nc4ccc(F)c(c4)C(F)(F)F)cc3)c(C)nc2cc1OCCN1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114434
BindingDB Entry DOI: 10.7270/Q2833X0F |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50541452
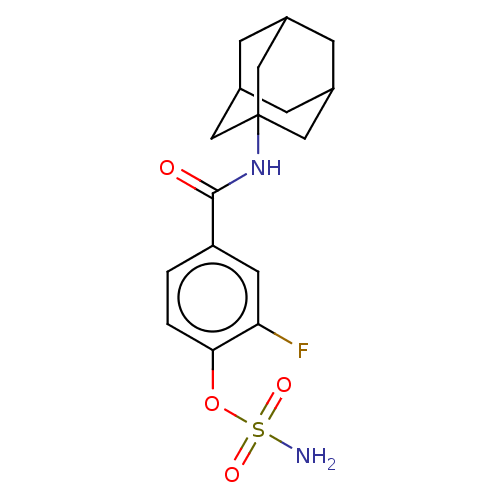
(CHEMBL4646243)Show SMILES NS(=O)(=O)Oc1ccc(cc1F)C(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:14:15:18:22.20.21,THB:20:19:16:22.21.23,20:21:18.19.24:16,23:21:18:24.15.16,23:15:18:22.20.21| Show InChI InChI=1S/C17H21FN2O4S/c18-14-6-13(1-2-15(14)24-25(19,22)23)16(21)20-17-7-10-3-11(8-17)5-12(4-10)9-17/h1-2,6,10-12H,3-5,7-9H2,(H,20,21)(H2,19,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell lysates using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK3 (unknown origin) incubated for 40 mins in presence of Mg/ATP mix by [gamma p33]-ATP based scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50541454

(CHEMBL4636936)Show SMILES NS(=O)(=O)Oc1ccc(cc1Br)C(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:14:15:18:22.20.21,THB:20:19:16:22.21.23,20:21:18.19.24:16,23:21:18:24.15.16,23:15:18:22.20.21| Show InChI InChI=1S/C17H21BrN2O4S/c18-14-6-13(1-2-15(14)24-25(19,22)23)16(21)20-17-7-10-3-11(8-17)5-12(4-10)9-17/h1-2,6,10-12H,3-5,7-9H2,(H,20,21)(H2,19,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell lysates using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50591873
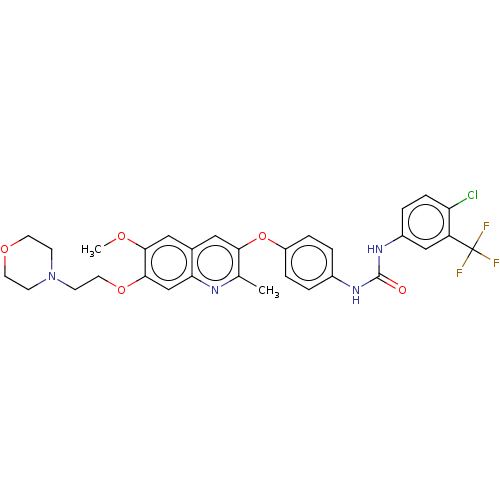
(CHEMBL5189396)Show SMILES COc1cc2cc(Oc3ccc(NC(=O)Nc4ccc(Cl)c(c4)C(F)(F)F)cc3)c(C)nc2cc1OCCN1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114434
BindingDB Entry DOI: 10.7270/Q2833X0F |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KDR (unknown origin) in presence of [gamma33]-ATP by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113081
BindingDB Entry DOI: 10.7270/Q2B85CV4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50562672
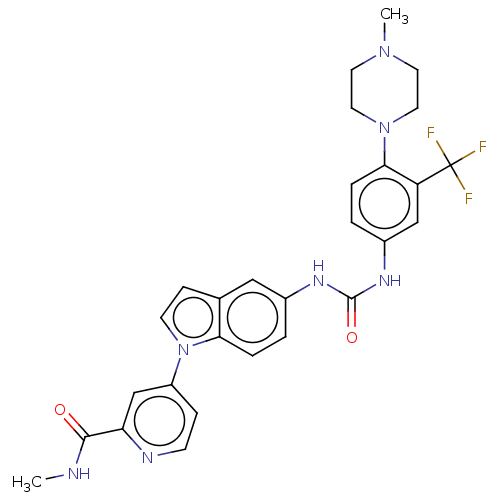
(CHEMBL4797840)Show SMILES CNC(=O)c1cc(ccn1)-n1ccc2cc(NC(=O)Nc3ccc(N4CCN(C)CC4)c(c3)C(F)(F)F)ccc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Flt4 (unknown origin) in presence of [gamma33]-ATP by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113081
BindingDB Entry DOI: 10.7270/Q2B85CV4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50541451

(CHEMBL4635151)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)C23CC4CC(CC(C4)C2)C3)cc1 |TLB:10:12:15:19.17.18,THB:17:16:13:19.18.20,17:18:15.16.21:13,20:18:15:21.12.13,20:12:15:19.17.18| Show InChI InChI=1S/C17H22N2O4S/c18-24(21,22)23-15-3-1-14(2-4-15)19-16(20)17-8-11-5-12(9-17)7-13(6-11)10-17/h1-4,11-13H,5-10H2,(H,19,20)(H2,18,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell lysates using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ErbB4 (unknown origin) incubated for 40 mins in presence of Mg/ATP mix by [gamma p33]-ATP based scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50524779

(CHEMBL4447079)Show SMILES Fc1ccc(cc1)S(=O)(=O)Oc1ccc(cc1)-c1ccc2occc2c1 Show InChI InChI=1S/C20H13FO4S/c21-17-4-8-19(9-5-17)26(22,23)25-18-6-1-14(2-7-18)15-3-10-20-16(13-15)11-12-24-20/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA-tagged carbonic anhydrase 2 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after 3... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50541453

(CHEMBL4637433)Show SMILES NS(=O)(=O)Oc1ccc(cc1Cl)C(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:14:15:18:22.20.21,THB:20:19:16:22.21.23,20:21:18.19.24:16,23:21:18:24.15.16,23:15:18:22.20.21| Show InChI InChI=1S/C17H21ClN2O4S/c18-14-6-13(1-2-15(14)24-25(19,22)23)16(21)20-17-7-10-3-11(8-17)5-12(4-10)9-17/h1-2,6,10-12H,3-5,7-9H2,(H,20,21)(H2,19,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate incubated for 20 hrs by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50524773
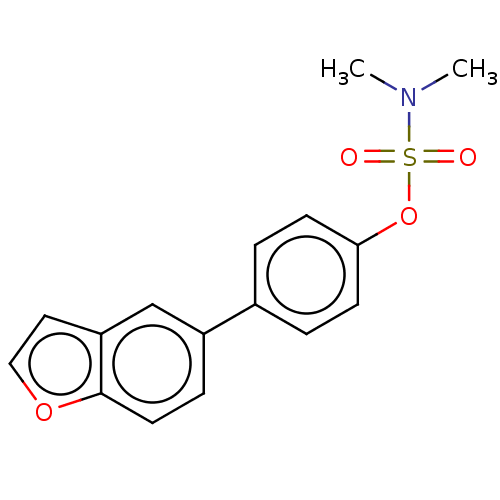
(CHEMBL4533978)Show InChI InChI=1S/C16H15NO4S/c1-17(2)22(18,19)21-15-6-3-12(4-7-15)13-5-8-16-14(11-13)9-10-20-16/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA--tagged carbonic anhydrase 12 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50524777
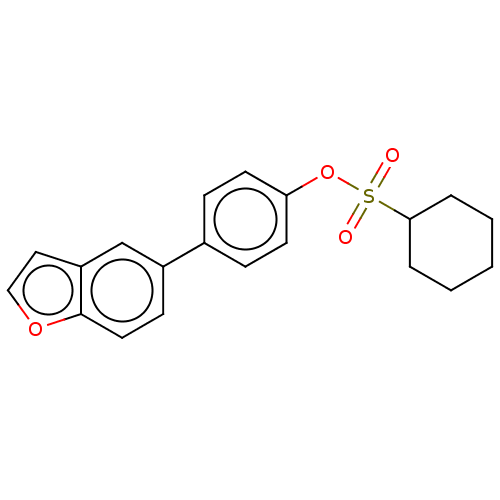
(CHEMBL4452931)Show InChI InChI=1S/C20H20O4S/c21-25(22,19-4-2-1-3-5-19)24-18-9-6-15(7-10-18)16-8-11-20-17(14-16)12-13-23-20/h6-14,19H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA--tagged carbonic anhydrase 12 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50562672

(CHEMBL4797840)Show SMILES CNC(=O)c1cc(ccn1)-n1ccc2cc(NC(=O)Nc3ccc(N4CCN(C)CC4)c(c3)C(F)(F)F)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KDR (unknown origin) in presence of [gamma33]-ATP by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113081
BindingDB Entry DOI: 10.7270/Q2B85CV4 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50569246
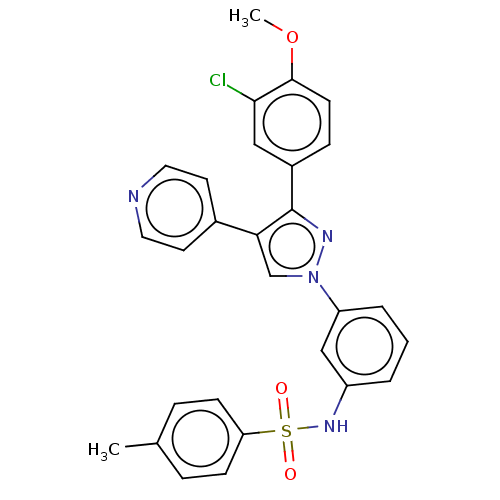
(CHEMBL4878720)Show SMILES COc1ccc(cc1Cl)-c1nn(cc1-c1ccncc1)-c1cccc(NS(=O)(=O)c2ccc(C)cc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of NPP1 (unknown origin) transfected in COS7 cells using pNP-TMP as substrate incubated for 35 mins by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113339
BindingDB Entry DOI: 10.7270/Q22R3WFJ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ErbB4 in human T47D cells assessed as suppression of neuregulin 1-induced autophosphorylation incubated for 90 mins by sandwich ELISA |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50591874
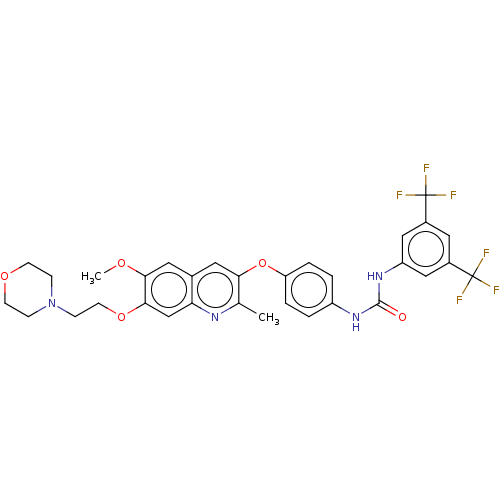
(CHEMBL5174740)Show SMILES COc1cc2cc(Oc3ccc(NC(=O)Nc4cc(cc(c4)C(F)(F)F)C(F)(F)F)cc3)c(C)nc2cc1OCCN1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114434
BindingDB Entry DOI: 10.7270/Q2833X0F |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
(Homo sapiens (Human)) | BDBM50569247
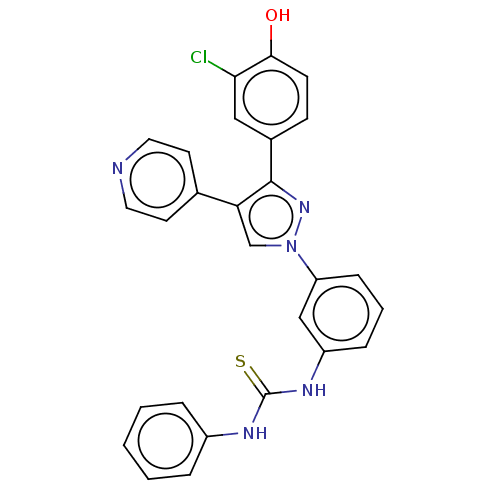
(CHEMBL4859945)Show SMILES Oc1ccc(cc1Cl)-c1nn(cc1-c1ccncc1)-c1cccc(NC(=S)Nc2ccccc2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of NPP3 (unknown origin) transfected in COS7 cells using pNP-TMP as substrate incubated for 35 mins by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113339
BindingDB Entry DOI: 10.7270/Q22R3WFJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50524780
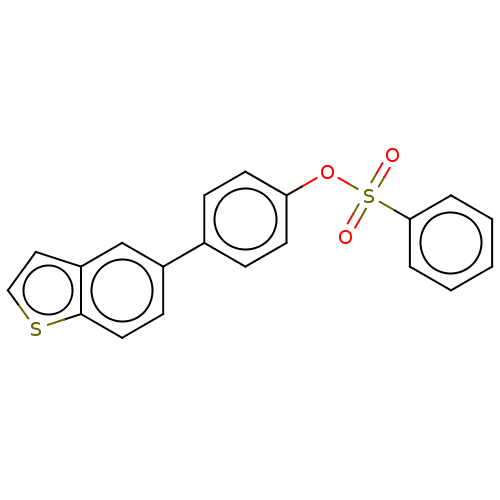
(CHEMBL4540118)Show InChI InChI=1S/C20H14O3S2/c21-25(22,19-4-2-1-3-5-19)23-18-9-6-15(7-10-18)16-8-11-20-17(14-16)12-13-24-20/h1-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA--tagged carbonic anhydrase 12 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50524779

(CHEMBL4447079)Show SMILES Fc1ccc(cc1)S(=O)(=O)Oc1ccc(cc1)-c1ccc2occc2c1 Show InChI InChI=1S/C20H13FO4S/c21-17-4-8-19(9-5-17)26(22,23)25-18-6-1-14(2-7-18)15-3-10-20-16(13-15)11-12-24-20/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA--tagged carbonic anhydrase 12 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50524768
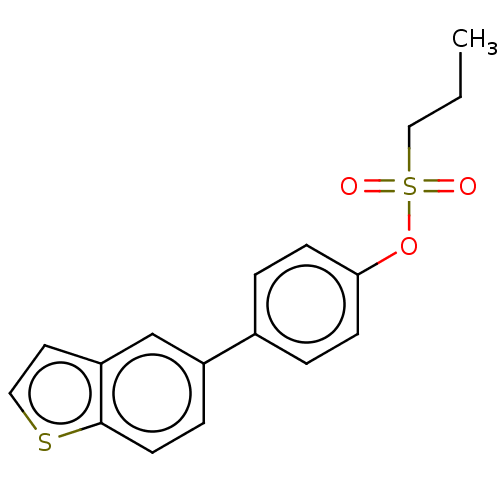
(CHEMBL4469527)Show InChI InChI=1S/C17H16O3S2/c1-2-11-22(18,19)20-16-6-3-13(4-7-16)14-5-8-17-15(12-14)9-10-21-17/h3-10,12H,2,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA--tagged carbonic anhydrase 12 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50524768

(CHEMBL4469527)Show InChI InChI=1S/C17H16O3S2/c1-2-11-22(18,19)20-16-6-3-13(4-7-16)14-5-8-17-15(12-14)9-10-21-17/h3-10,12H,2,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA-tagged carbonic anhydrase 2 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after 3... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50232424
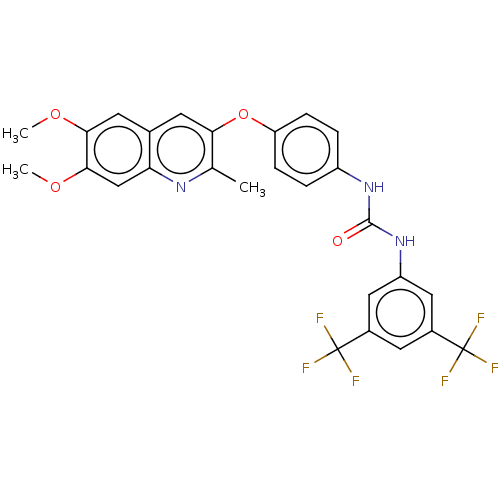
(CHEMBL4097123)Show SMILES COc1cc2cc(Oc3ccc(NC(=O)Nc4cc(cc(c4)C(F)(F)F)C(F)(F)F)cc3)c(C)nc2cc1OC Show InChI InChI=1S/C27H21F6N3O4/c1-14-22(8-15-9-23(38-2)24(39-3)13-21(15)34-14)40-20-6-4-18(5-7-20)35-25(37)36-19-11-16(26(28,29)30)10-17(12-19)27(31,32)33/h4-13H,1-3H3,(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114434
BindingDB Entry DOI: 10.7270/Q2833X0F |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50574708

(CHEMBL4866102)Show SMILES C(Oc1cccc(c1)-c1nc2sccn2c1-c1ccncn1)c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR- T790M mutant (unknown origin) incubated for 40 mins in presence of Mg/ATP mix by [gamma p33]-ATP based scintillation counting met... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50524770
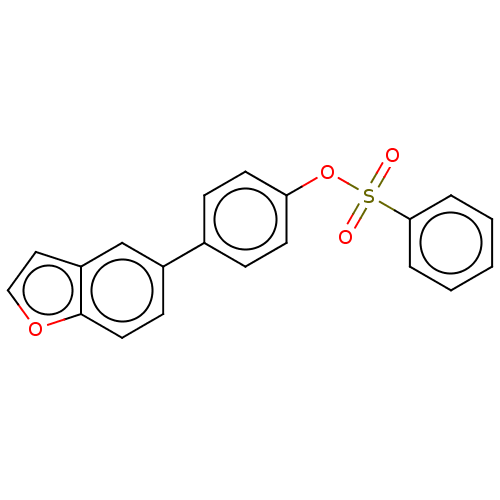
(CHEMBL4554647)Show InChI InChI=1S/C20H14O4S/c21-25(22,19-4-2-1-3-5-19)24-18-9-6-15(7-10-18)16-8-11-20-17(14-16)12-13-23-20/h1-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA-tagged carbonic anhydrase 2 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after 3... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 427 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDGFRalpha (unknown origin) in presence of [gamma33]-ATP by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113081
BindingDB Entry DOI: 10.7270/Q2B85CV4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50524770

(CHEMBL4554647)Show InChI InChI=1S/C20H14O4S/c21-25(22,19-4-2-1-3-5-19)24-18-9-6-15(7-10-18)16-8-11-20-17(14-16)12-13-23-20/h1-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA--tagged carbonic anhydrase 12 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50524771

(CHEMBL4517453)Show SMILES Cc1ccc(cc1)S(=O)(=O)Oc1ccc(cc1)-c1ccc2occc2c1 Show InChI InChI=1S/C21H16O4S/c1-15-2-9-20(10-3-15)26(22,23)25-19-7-4-16(5-8-19)17-6-11-21-18(14-17)12-13-24-21/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA-tagged carbonic anhydrase 2 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after 3... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50524769
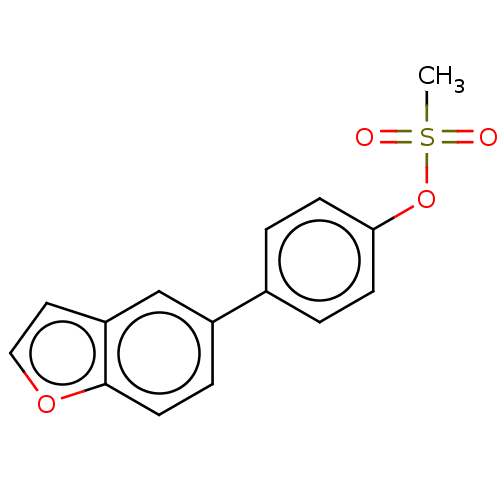
(CHEMBL4592008)Show InChI InChI=1S/C15H12O4S/c1-20(16,17)19-14-5-2-11(3-6-14)12-4-7-15-13(10-12)8-9-18-15/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA--tagged carbonic anhydrase 12 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
(Homo sapiens (Human)) | BDBM50569257
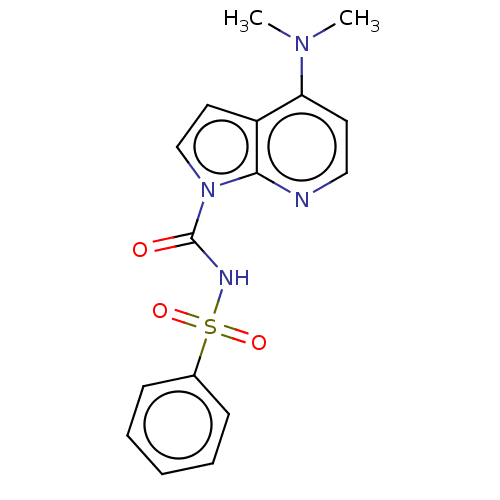
(CHEMBL4848343)Show SMILES CN(C)c1ccnc2n(ccc12)C(=O)NS(=O)(=O)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NPP3 transfected in COS7 cells using pNP-TMP as substrate incubated for 35 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113339
BindingDB Entry DOI: 10.7270/Q22R3WFJ |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
(Homo sapiens (Human)) | BDBM50569253
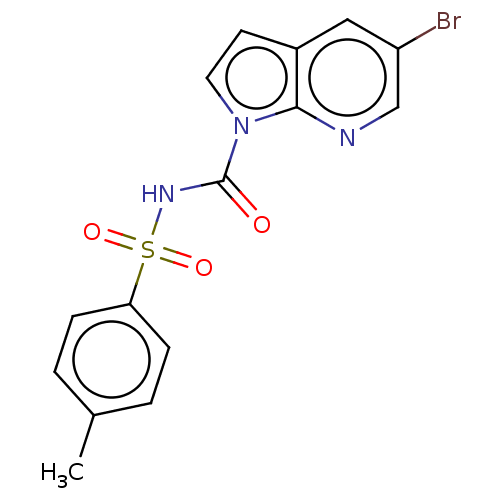
(CHEMBL4850521)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)n1ccc2cc(Br)cnc12 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NPP3 transfected in COS7 cells using pNP-TMP as substrate incubated for 35 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113339
BindingDB Entry DOI: 10.7270/Q22R3WFJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50574708

(CHEMBL4866102)Show SMILES C(Oc1cccc(c1)-c1nc2sccn2c1-c1ccncn1)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KDR (unknown origin) incubated for 40 mins in presence of Mg/ATP mix by [gamma p33]-ATP based scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50569250
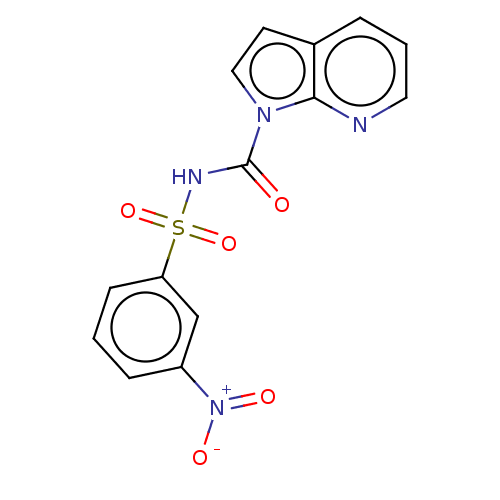
(CHEMBL4875454)Show SMILES [O-][N+](=O)c1cccc(c1)S(=O)(=O)NC(=O)n1ccc2cccnc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NPP1 transfected in COS7 cells using pNP-TMP as substrate incubated for 35 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113339
BindingDB Entry DOI: 10.7270/Q22R3WFJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50524767
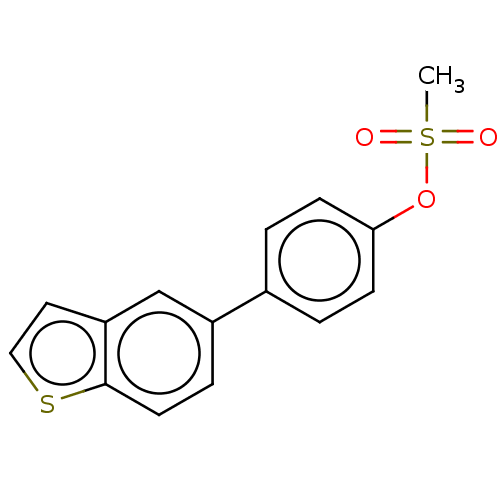
(CHEMBL4591628)Show InChI InChI=1S/C15H12O3S2/c1-20(16,17)18-14-5-2-11(3-6-14)12-4-7-15-13(10-12)8-9-19-15/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-HA--tagged carbonic anhydrase 12 expressed in HEK293T cells using p-nitrophenyl acetate as substrate measured after... |
Bioorg Med Chem 27: 3889-3901 (2019)
Article DOI: 10.1016/j.bmc.2019.07.026
BindingDB Entry DOI: 10.7270/Q2GX4G0K |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
(Homo sapiens (Human)) | BDBM50569249
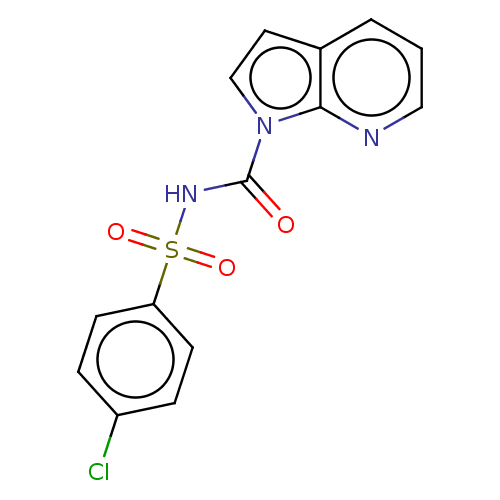
(CHEMBL4859229) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NPP3 transfected in COS7 cells using pNP-TMP as substrate incubated for 35 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113339
BindingDB Entry DOI: 10.7270/Q22R3WFJ |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
(Homo sapiens (Human)) | BDBM50179360

(CHEMBL3040216)Show SMILES CC1=CCC(=C\C1=N\C(=O)C1=CC=C\C(C1)=N/C(=O)/N=C1/CC(=CC=C1)C(=O)\N=C1\CC(=CC=C1C)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O |c:4,13,24,26,34,36,45,72,t:1,11| Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-11,15-16,20-24H,12-14,17-19H2,1-2H3,(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)/b52-31+,53-32+,54-37+,55-38+,56-39-,57-40- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NPP3 transfected in COS7 cells using pNP-TMP as substrate incubated for 35 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113339
BindingDB Entry DOI: 10.7270/Q22R3WFJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data