

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118238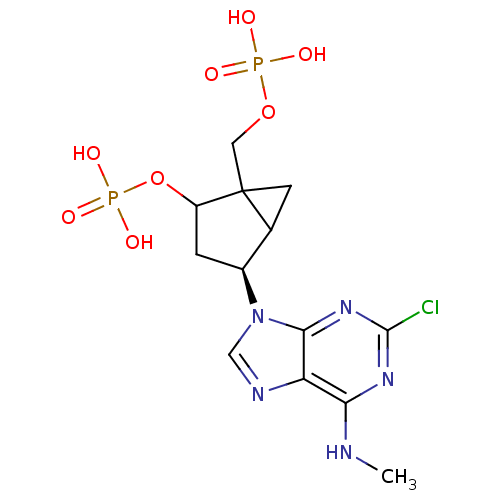 (CHEMBL339873 | MRS 2279) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against Turkey erythrocyte P2Y purinoceptor 1 (P2Y1) by the compound is measured | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50076460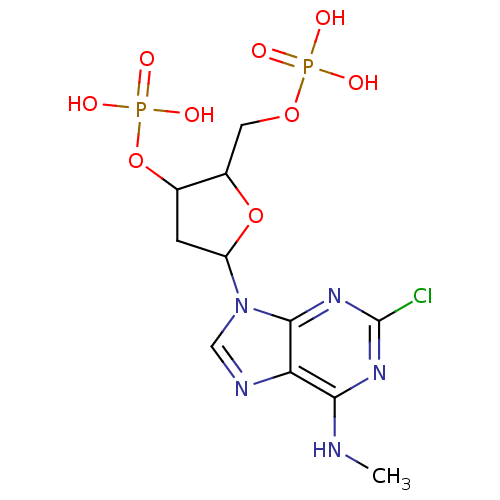 (CHEMBL288798 | Phosphoric acid mono-[5-(2-chloro-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against Turkey erythrocyte P2Y purinoceptor 1 (P2Y1) by the compound is measured | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50409486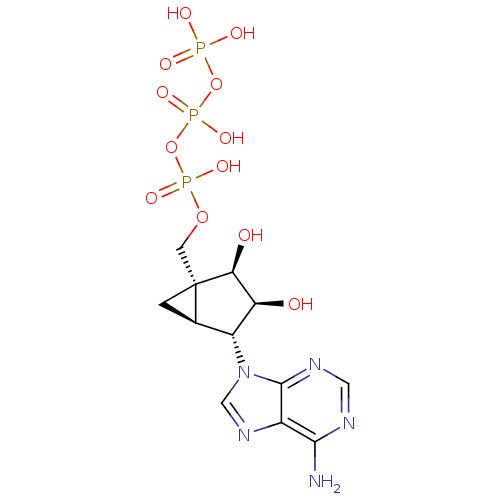 (CHEMBL2111533) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 2 (hP2Y2) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM18137 (AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 1 (hP2Y1) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM18137 (AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 1 (hP2Y1) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM18137 (AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 2 (hP2Y2) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50409486 (CHEMBL2111533) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 1 (hP2Y1) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50306712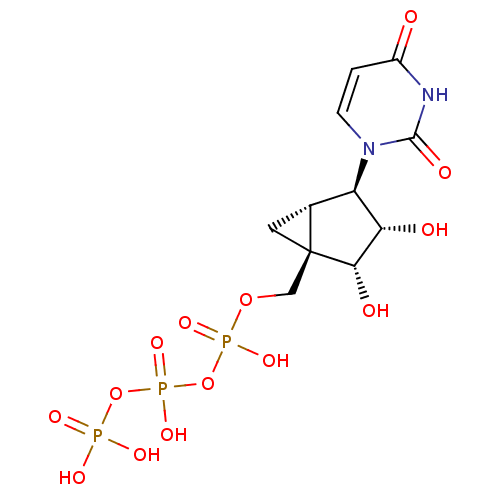 (((1R,2R,3S,4R,5S)-4-(2,4-dioxo-3,4-dihydropyrimidi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 4 (hP2Y4) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50409486 (CHEMBL2111533) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 11 (hP2Y11) stably expressed in 131N1 astrocytoma cell at 10 uM | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50403871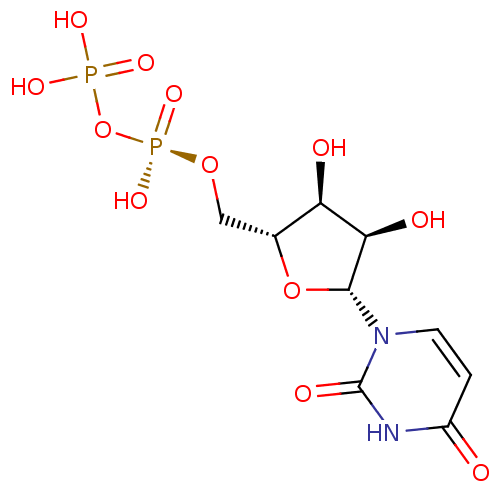 (URIDINE_DIPHOSPHATE) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 6 (hP2Y6) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50409486 (CHEMBL2111533) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 91 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 2 (hP2Y2) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50409486 (CHEMBL2111533) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at turkey Purinoceptor P2Y1 stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50306712 (((1R,2R,3S,4R,5S)-4-(2,4-dioxo-3,4-dihydropyrimidi...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 15.9 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 2 (hP2Y2) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM18137 (AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at turkey Purinoceptor P2Y1 stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50306712 (((1R,2R,3S,4R,5S)-4-(2,4-dioxo-3,4-dihydropyrimidi...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 15.9 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 2 (hP2Y2) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50409486 (CHEMBL2111533) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at turkey Purinoceptor P2Y1 stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50409486 (CHEMBL2111533) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 1 (hP2Y1) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM18137 (AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at turkey Purinoceptor P2Y1 stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50306712 (((1R,2R,3S,4R,5S)-4-(2,4-dioxo-3,4-dihydropyrimidi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 4 (hP2Y4) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM18137 (AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 11 (hP2Y11) stably expressed in 131N1 astrocytoma cell at 10 uM | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM18137 (AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 2 (hP2Y2) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||