

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13433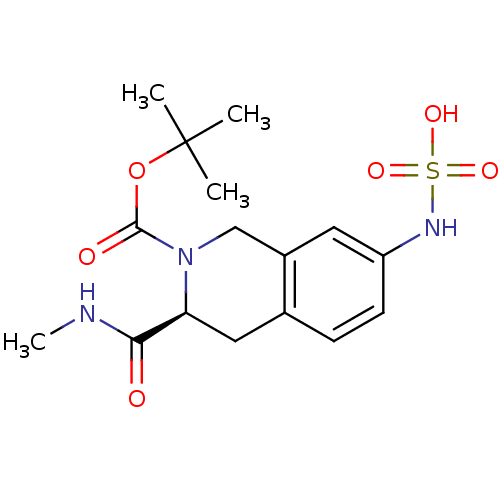 (N-[(3S)-2-[(tert-butoxy)carbonyl]-3-(methylcarbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 2.40E+4 | -26.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Procter & Gamble Pharmaceuticals | Assay Description The activity of PTP1B enzyme was assayed with DiFMUP as substrate. Hydrolysis of substrate was monitored on a Victor V plate reader (Wallac). Kinetic... | Bioorg Med Chem Lett 16: 1574-8 (2006) Article DOI: 10.1016/j.bmcl.2005.12.051 BindingDB Entry DOI: 10.7270/Q2PN93WP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13529 (N-(1-methyl-3-phenyl-1H-pyrazol-5-yl)sulfamic acid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG MMDB PC cid PC sid PDB UniChem | DrugBank MMDB PDB Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals | Assay Description The activity of PTP1B enzyme was assayed with DiFMUP as substrate. Hydrolysis of substrate was monitored on a Victor V plate reader (Wallac). Kinetic... | Bioorg Med Chem Lett 16: 1574-8 (2006) Article DOI: 10.1016/j.bmcl.2005.12.051 BindingDB Entry DOI: 10.7270/Q2PN93WP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188792 (CHEMBL213750 | ammonium N-{4-[3-methoxy-2-(methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188795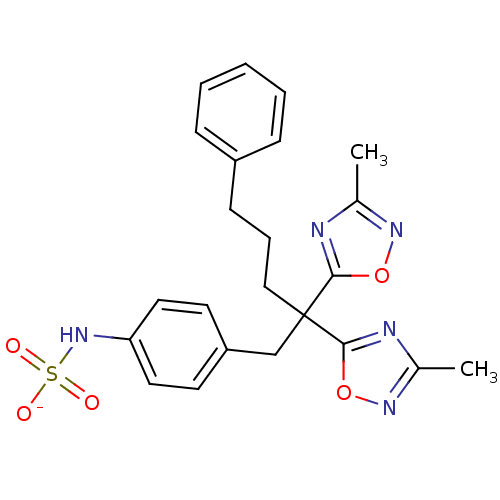 (CHEMBL386550 | ammonium N-{4-[2,2-bis(3-methyl-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188791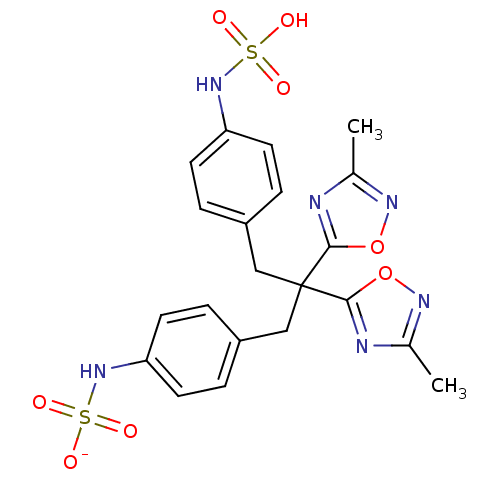 (CHEMBL213732 | ammonium N-{4-[2,2-bis(3-methyl-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188775 (CHEMBL214192 | ammonium N-{4-[3-methoxy-2-(methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107703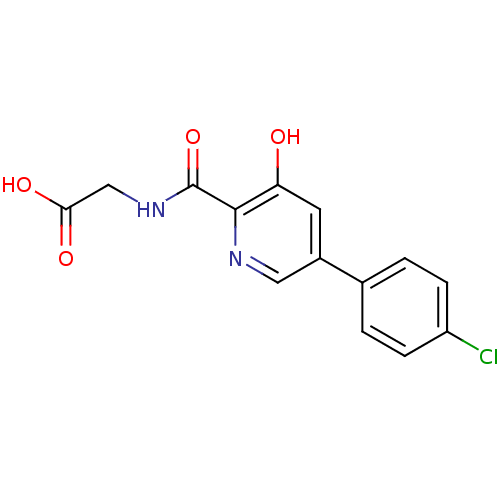 (US10889546, US2007/0299086 Table 2.4 | US11426393,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description HEK293 cells are seeded in 96-well poly-lysine coated plates at 20,000 cells per well in DMEM (10% FBS, 1% NEAA, 0.1% glutamine). Following overnight... | US Patent US9598370 (2017) BindingDB Entry DOI: 10.7270/Q20G3N64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107703 (US10889546, US2007/0299086 Table 2.4 | US11426393,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | 25 |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8722895 (2014) BindingDB Entry DOI: 10.7270/Q25Q4TRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107703 (US10889546, US2007/0299086 Table 2.4 | US11426393,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8598210 (2013) BindingDB Entry DOI: 10.7270/Q2M04428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107703 (US10889546, US2007/0299086 Table 2.4 | US11426393,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107703 (US10889546, US2007/0299086 Table 2.4 | US11426393,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Los Angeles | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Bioorg Med Chem 17: 7174-85 (2009) BindingDB Entry DOI: 10.7270/Q2D50Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188801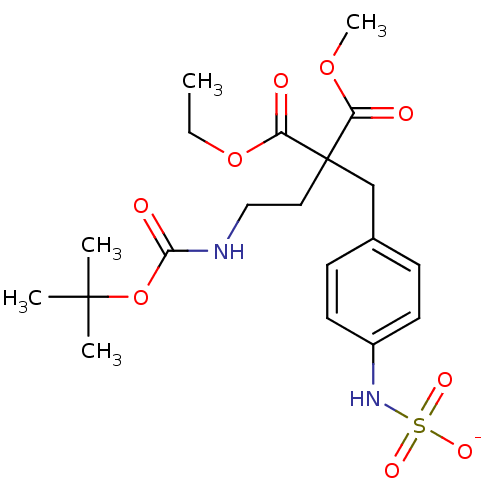 (CHEMBL213620 | ammonium N-{4-[2-(2-{[(tert-butoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107702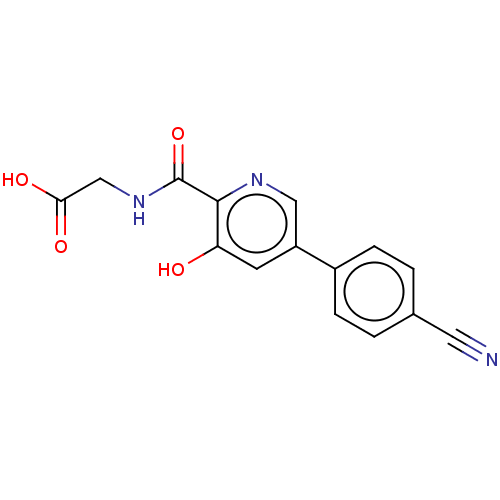 (US8598210, Table XV, 8 | US8722895, 8: {[5-(4-Cyan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8598210 (2013) BindingDB Entry DOI: 10.7270/Q2M04428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188797 (CHEMBL213802 | ammonium N-{4-[3-methoxy-2-(methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188781 (CHEMBL209527 | ammonium N-(4-{2-[(4-carboxyphenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188780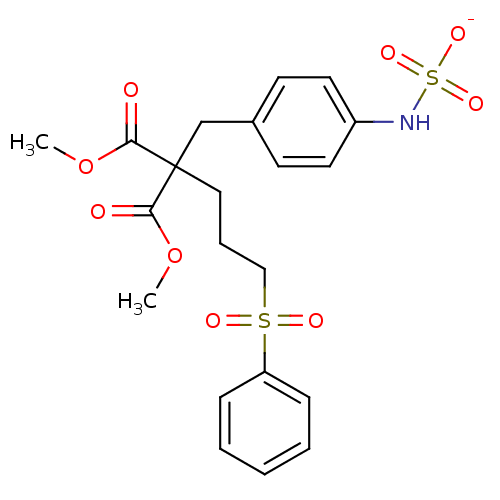 (CHEMBL211786 | ammonium N-(4-{2-[3-(benzenesulfony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188784 (CHEMBL385251 | ammonium N-{4-[3-methoxy-2-(methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107698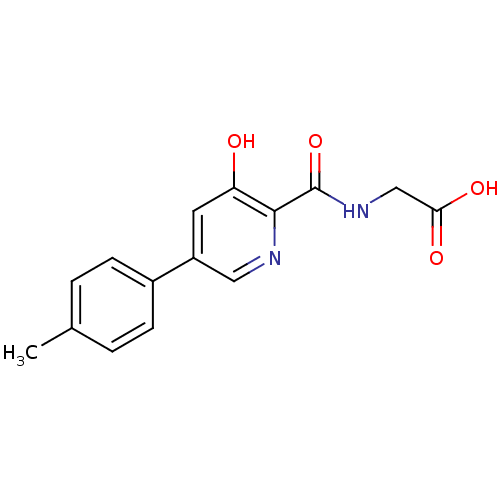 (US11426393, Compound Table XV.4 | US8598210, Table...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description HEK293 cells are seeded in 96-well poly-lysine coated plates at 20,000 cells per well in DMEM (10% FBS, 1% NEAA, 0.1% glutamine). Following overnight... | US Patent US9598370 (2017) BindingDB Entry DOI: 10.7270/Q20G3N64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107698 (US11426393, Compound Table XV.4 | US8598210, Table...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | 25 |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8722895 (2014) BindingDB Entry DOI: 10.7270/Q25Q4TRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107698 (US11426393, Compound Table XV.4 | US8598210, Table...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8598210 (2013) BindingDB Entry DOI: 10.7270/Q2M04428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107698 (US11426393, Compound Table XV.4 | US8598210, Table...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Los Angeles | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Bioorg Med Chem 17: 7174-85 (2009) BindingDB Entry DOI: 10.7270/Q2D50Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107698 (US11426393, Compound Table XV.4 | US8598210, Table...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM400041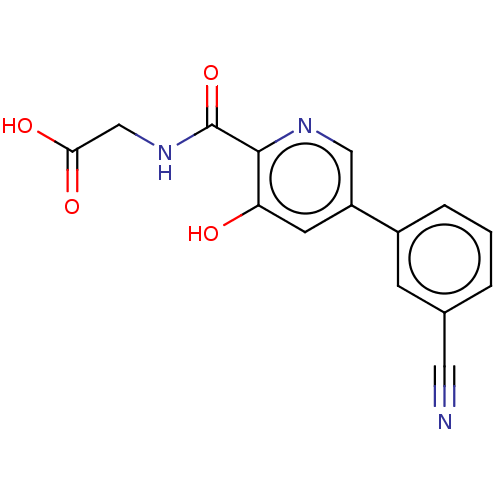 (US11426393, Compound Table XV.20 | USRE47437, Exam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Los Angeles | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Bioorg Med Chem 17: 7174-85 (2009) BindingDB Entry DOI: 10.7270/Q2D50Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [179-426] (Homo sapiens (Human)) | BDBM400041 (US11426393, Compound Table XV.20 | USRE47437, Exam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107714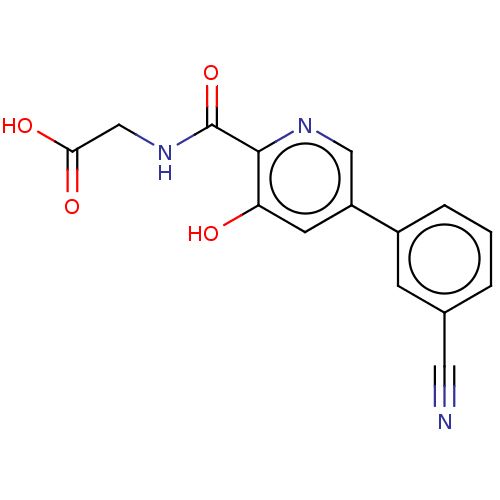 (US8598210, Table XV, 20 | US8722895, 20: {[5-(3-Cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | 25 |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8722895 (2014) BindingDB Entry DOI: 10.7270/Q25Q4TRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107714 (US8598210, Table XV, 20 | US8722895, 20: {[5-(3-Cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US9598370 (2017) BindingDB Entry DOI: 10.7270/Q20G3N64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107714 (US8598210, Table XV, 20 | US8722895, 20: {[5-(3-Cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8598210 (2013) BindingDB Entry DOI: 10.7270/Q2M04428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50370782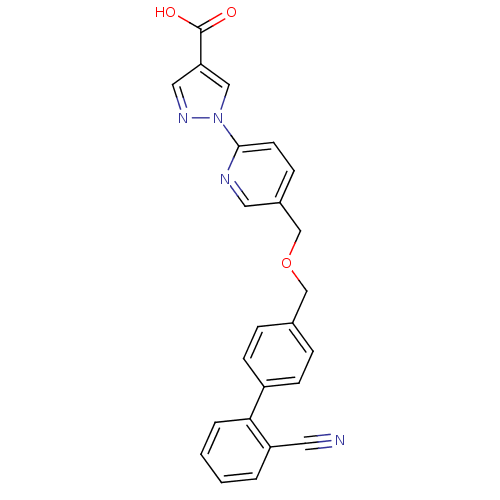 (CHEMBL222115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human EGLN1 | Bioorg Med Chem Lett 16: 5687-90 (2006) Article DOI: 10.1016/j.bmcl.2006.08.017 BindingDB Entry DOI: 10.7270/Q25Q4WXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188778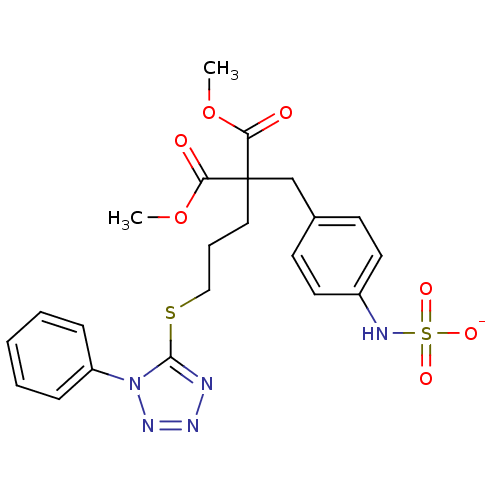 (CHEMBL213626 | ammonium N-{4-[3-methoxy-2-(methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188782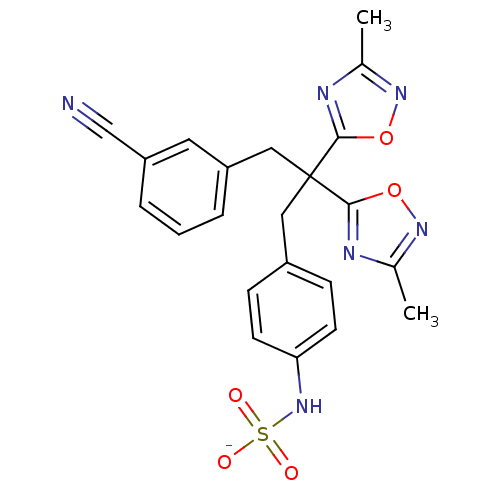 (CHEMBL384201 | ammonium N-{4-[3-(3-cyanophenyl)-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188779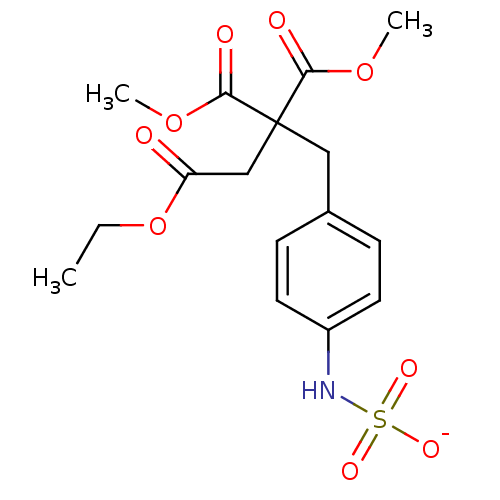 (CHEMBL378423 | ammonium N-{4-[4-ethoxy-2,2-bis(met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188783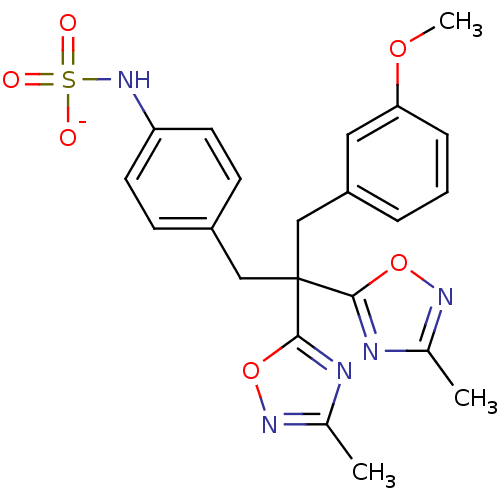 (CHEMBL213226 | ammonium N-{4-[3-(3-methoxyphenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107703 (US10889546, US2007/0299086 Table 2.4 | US11426393,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US9598370 (2017) BindingDB Entry DOI: 10.7270/Q20G3N64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107703 (US10889546, US2007/0299086 Table 2.4 | US11426393,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Los Angeles | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Bioorg Med Chem 17: 7174-85 (2009) BindingDB Entry DOI: 10.7270/Q2D50Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [179-426] (Homo sapiens (Human)) | BDBM107703 (US10889546, US2007/0299086 Table 2.4 | US11426393,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107703 (US10889546, US2007/0299086 Table 2.4 | US11426393,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | 25 |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8722895 (2014) BindingDB Entry DOI: 10.7270/Q25Q4TRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107703 (US10889546, US2007/0299086 Table 2.4 | US11426393,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8598210 (2013) BindingDB Entry DOI: 10.7270/Q2M04428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM17854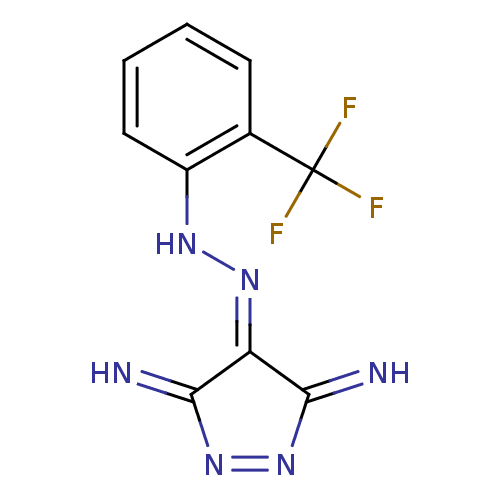 (3,5-Pyrazolediamine Inhibitor, 4 | 3-imino-4-[(E)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 7.5 | 30 |
The Procter & Gamble Pharmaceuticals | Assay Description The inhibitory activity of a compound toward EcMAP was measured by incubating the compound at various concentrations in the presence of the enzyme an... | Proteins 66: 538-46 (2007) Article DOI: 10.1002/prot.21207 BindingDB Entry DOI: 10.7270/Q2MK6B5Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM17853 (3,5-Pyrazolediamine Inhibitor, 3 | 3-imino-4-[(E)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 7.5 | 30 |
The Procter & Gamble Pharmaceuticals | Assay Description The inhibitory activity of a compound toward EcMAP was measured by incubating the compound at various concentrations in the presence of the enzyme an... | Proteins 66: 538-46 (2007) Article DOI: 10.1002/prot.21207 BindingDB Entry DOI: 10.7270/Q2MK6B5Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188798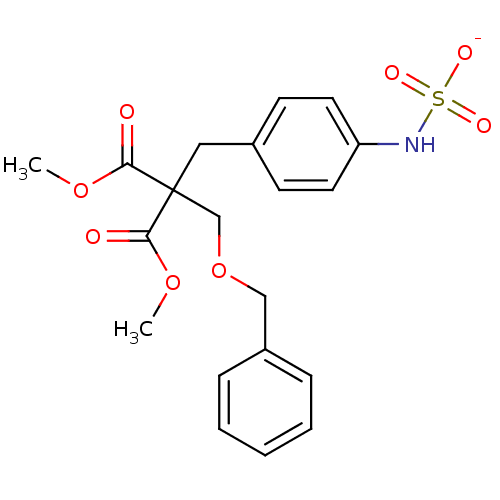 (CHEMBL385818 | ammonium N-(4-{2-[(benzyloxy)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188794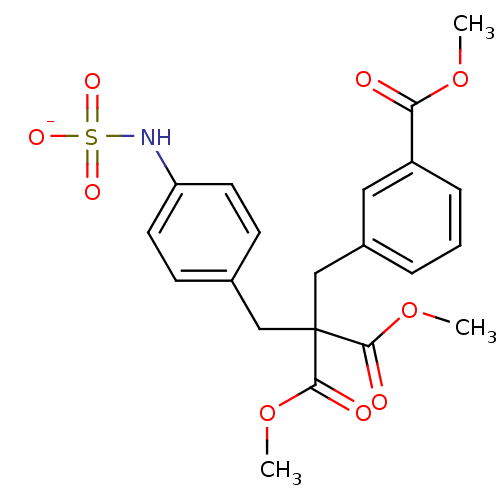 (CHEMBL380374 | ammonium N-{4-[3-methoxy-2-(methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107717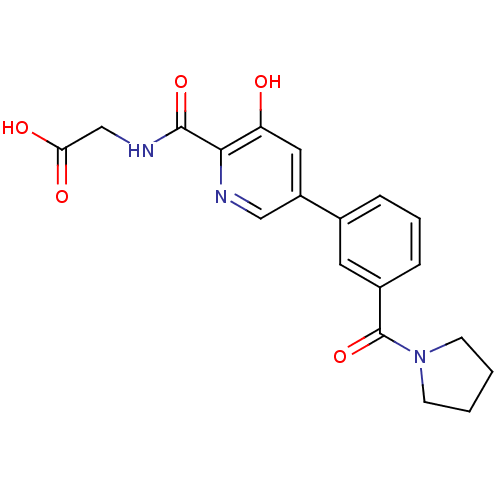 (US11426393, Compound Table XV.23 | US8598210, Tabl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Los Angeles | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Bioorg Med Chem 17: 7174-85 (2009) BindingDB Entry DOI: 10.7270/Q2D50Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107708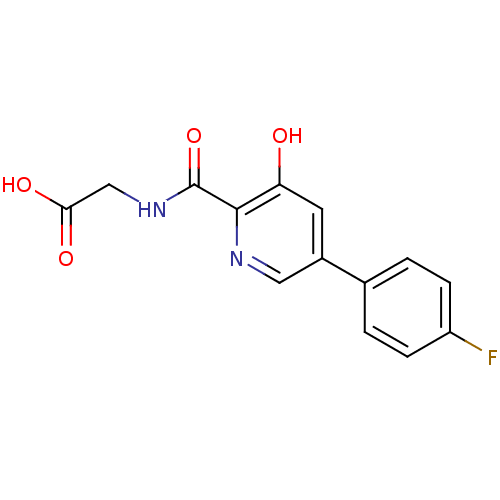 (US11426393, Compound Table XV.14 | US8598210, Tabl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8598210 (2013) BindingDB Entry DOI: 10.7270/Q2M04428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50188793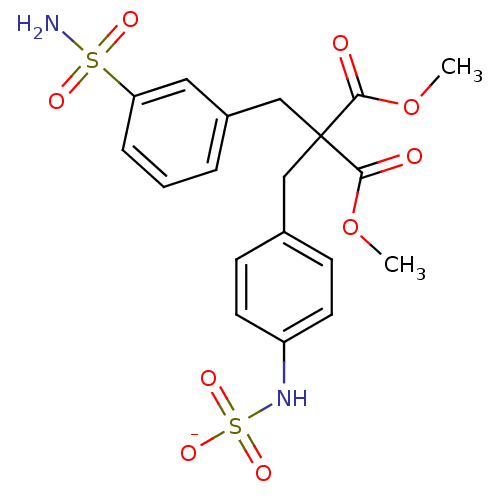 (CHEMBL209957 | ammonium N-{4-[3-methoxy-2-(methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTPbeta | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107708 (US11426393, Compound Table XV.14 | US8598210, Tabl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | 25 |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8722895 (2014) BindingDB Entry DOI: 10.7270/Q25Q4TRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107717 (US11426393, Compound Table XV.23 | US8598210, Tabl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | 25 |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8722895 (2014) BindingDB Entry DOI: 10.7270/Q25Q4TRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107708 (US11426393, Compound Table XV.14 | US8598210, Tabl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Los Angeles | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Bioorg Med Chem 17: 7174-85 (2009) BindingDB Entry DOI: 10.7270/Q2D50Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [179-426] (Homo sapiens (Human)) | BDBM107708 (US11426393, Compound Table XV.14 | US8598210, Tabl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107717 (US11426393, Compound Table XV.23 | US8598210, Tabl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US9598370 (2017) BindingDB Entry DOI: 10.7270/Q20G3N64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM107717 (US11426393, Compound Table XV.23 | US8598210, Tabl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8598210 (2013) BindingDB Entry DOI: 10.7270/Q2M04428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 425 total ) | Next | Last >> |