

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50345191 (7,8-Benzo-4'-hydroxy-4-imidazolylflavan | CHEMBL17...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50345190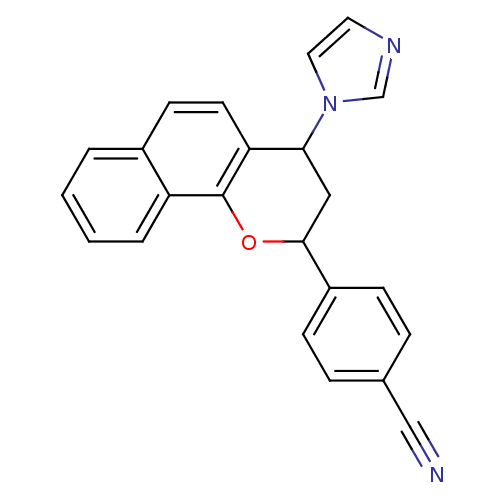 (2,4-cis-7,8-benzo-4'-cyano-4-imidazolylflavan | 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50345189 (2,4-trans-7,8-benzo-4-imidazolylflavan | CHEMBL178...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50118966 (1-(7-Methoxy-2-phenyl-chroman-4-yl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50118966 (1-(7-Methoxy-2-phenyl-chroman-4-yl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50118966 (1-(7-Methoxy-2-phenyl-chroman-4-yl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50345190 (2,4-cis-7,8-benzo-4'-cyano-4-imidazolylflavan | 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 534 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50345190 (2,4-cis-7,8-benzo-4'-cyano-4-imidazolylflavan | 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 534 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50345188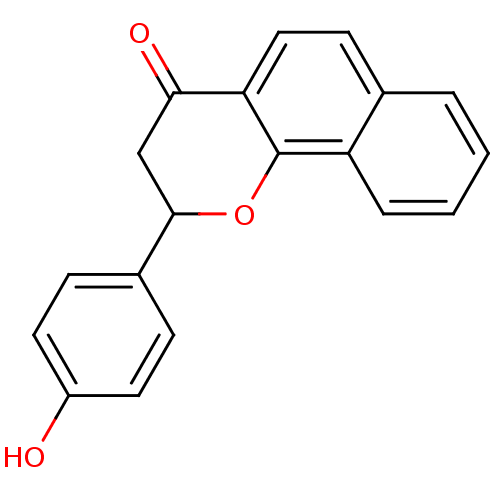 (2-(4-hydroxyphenyl)-2,3-dihydrobenzo[h]chromen-4-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50345187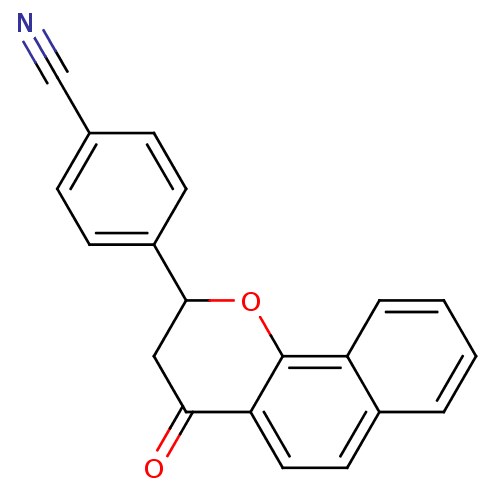 (4-(4-oxo-3,4-dihydro-2H-benzo[h]chromen-2-yl)benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50345186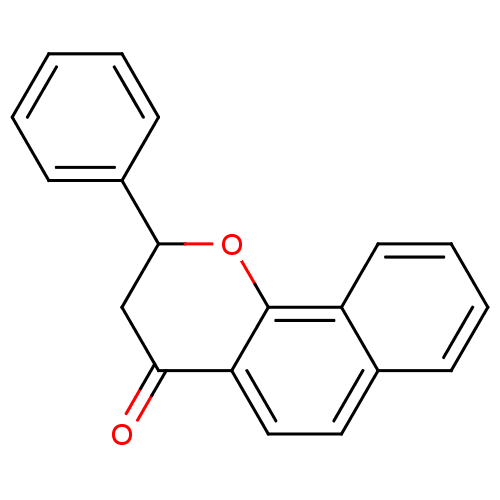 (2-phenyl-2,3-dihydrobenzo[h]chromen-4-one | 2-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES EA 4021 Biomol�cules et Th�rapies anti-tumorales Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as inhibition of aromatization of [1,2,6,7-3H] androstenedione by flow scintillation a... | Eur J Med Chem 46: 2541-5 (2011) Article DOI: 10.1016/j.ejmech.2011.03.043 BindingDB Entry DOI: 10.7270/Q2MC90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||