 Found 177 hits with Last Name = 'farjot' and Initial = 'g'
Found 177 hits with Last Name = 'farjot' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433406
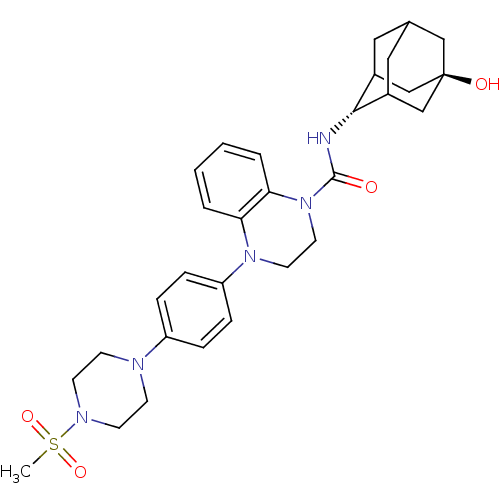
(CHEMBL2380643)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(1.07,-35.62,;1.84,-36.95,;1.84,-38.49,;.51,-37.71,;3.39,-36.95,;4.15,-38.27,;5.69,-38.27,;6.46,-36.95,;5.69,-35.62,;4.15,-35.62,;7.99,-36.94,;8.76,-38.28,;10.3,-38.28,;11.07,-36.95,;10.29,-35.61,;8.76,-35.61,;12.61,-36.94,;13.38,-38.28,;14.91,-38.29,;15.69,-36.95,;17.23,-36.96,;18,-38.29,;18.15,-35.73,;19.69,-35.75,;20.88,-34.48,;20.87,-32.99,;22.22,-32.51,;21.18,-33.75,;21.19,-35.33,;22.59,-35.9,;23.61,-34.62,;25.15,-34.62,;23.62,-33.09,;22.21,-34.97,;14.92,-35.62,;15.7,-34.29,;14.94,-32.95,;13.38,-32.94,;12.61,-34.28,;13.37,-35.61,)| Show InChI InChI=1S/C30H39N5O4S/c1-40(38,39)33-12-10-32(11-13-33)24-6-8-25(9-7-24)34-14-15-35(27-5-3-2-4-26(27)34)29(36)31-28-22-16-21-17-23(28)20-30(37,18-21)19-22/h2-9,21-23,28,37H,10-20H2,1H3,(H,31,36)/t21?,22?,23?,28-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433404

(CHEMBL2380641)Show SMILES CS(=O)(=O)N1CCN(CC1)c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(2.53,-27.78,;3.31,-29.1,;3.3,-30.64,;1.97,-29.87,;4.85,-29.11,;5.62,-30.43,;7.15,-30.43,;7.92,-29.1,;7.15,-27.77,;5.61,-27.78,;9.45,-29.1,;10.22,-30.44,;11.76,-30.44,;12.53,-29.1,;11.75,-27.76,;10.22,-27.77,;14.07,-29.1,;14.84,-30.44,;16.37,-30.44,;17.15,-29.11,;18.69,-29.12,;19.46,-30.45,;19.61,-27.88,;21.15,-27.91,;22.34,-26.63,;22.33,-25.15,;23.68,-24.67,;22.64,-25.9,;22.65,-27.49,;24.05,-28.05,;25.07,-26.78,;26.61,-26.78,;25.08,-25.25,;23.67,-27.12,;16.38,-27.78,;17.16,-26.45,;16.4,-25.1,;14.84,-25.1,;14.07,-26.43,;14.84,-27.77,)| Show InChI InChI=1S/C28H37N7O4S/c1-40(38,39)33-8-6-32(7-9-33)22-17-29-26(30-18-22)34-10-11-35(24-5-3-2-4-23(24)34)27(36)31-25-20-12-19-13-21(25)16-28(37,14-19)15-20/h2-5,17-21,25,37H,6-16H2,1H3,(H,31,36)/t19?,20?,21?,25-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433405

(CHEMBL2380642)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(25.74,-29.77,;26.51,-31.1,;26.5,-32.64,;25.17,-31.86,;28.05,-31.1,;28.82,-32.42,;30.36,-32.43,;31.12,-31.1,;30.35,-29.77,;28.81,-29.77,;32.66,-31.09,;33.42,-32.43,;34.96,-32.43,;35.73,-31.1,;34.96,-29.76,;33.42,-29.76,;37.28,-31.1,;38.05,-32.43,;39.58,-32.44,;40.35,-31.11,;41.89,-31.11,;42.66,-32.45,;42.82,-29.88,;44.35,-29.9,;45.55,-28.63,;45.54,-27.14,;46.89,-26.66,;45.85,-27.9,;45.85,-29.48,;47.26,-30.05,;48.27,-28.77,;49.81,-28.77,;48.28,-27.24,;46.88,-29.12,;39.59,-29.77,;40.37,-28.44,;39.6,-27.1,;38.05,-27.09,;37.28,-28.43,;38.04,-29.76,)| Show InChI InChI=1S/C29H38N6O4S/c1-40(38,39)33-10-8-32(9-11-33)23-6-7-26(30-19-23)34-12-13-35(25-5-3-2-4-24(25)34)28(36)31-27-21-14-20-15-22(27)18-29(37,16-20)17-21/h2-7,19-22,27,37H,8-18H2,1H3,(H,31,36)/t20?,21?,22?,27-,29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433411
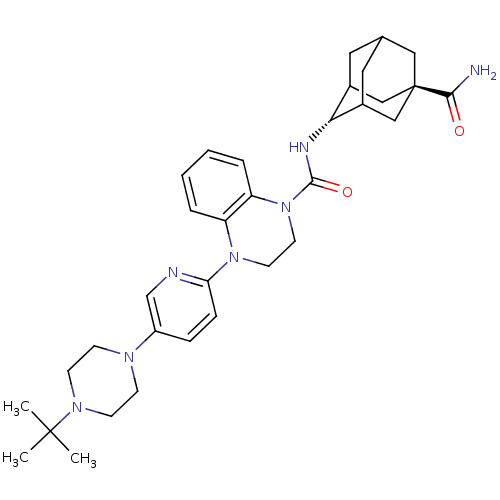
(CHEMBL2380648)Show SMILES CC(C)(C)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:23.24,wD:30.37,TLB:33:30:23.28.27:25,22:23:30.32.29:27.26.25,22:23:25:30.32.31,THB:29:30:23.28.27:25,29:28:25:30.32.31,31:30:23:27.26.25,31:26:23:30.32.29,(27.21,-52.3,;27.98,-53.63,;27.21,-54.97,;26.44,-53.63,;29.52,-53.63,;30.29,-54.95,;31.82,-54.95,;32.59,-53.63,;31.82,-52.3,;30.28,-52.3,;34.12,-53.62,;34.89,-52.29,;36.42,-52.29,;37.2,-53.63,;36.43,-54.96,;34.89,-54.96,;38.74,-53.62,;39.51,-54.96,;41.04,-54.97,;41.82,-53.63,;43.36,-53.64,;44.13,-54.97,;44.28,-52.41,;45.81,-52.44,;47.01,-51.16,;47,-49.68,;48.35,-49.2,;47.31,-50.43,;47.31,-52.02,;48.72,-52.58,;49.73,-51.3,;49.74,-49.78,;48.34,-51.65,;51.27,-51.3,;52.04,-52.64,;52.04,-49.97,;41.05,-52.3,;41.83,-50.97,;41.07,-49.63,;39.51,-49.62,;38.74,-50.96,;39.51,-52.29,)| Show InChI InChI=1S/C33H45N7O2/c1-32(2,3)38-12-10-37(11-13-38)25-8-9-28(35-21-25)39-14-15-40(27-7-5-4-6-26(27)39)31(42)36-29-23-16-22-17-24(29)20-33(18-22,19-23)30(34)41/h4-9,21-24,29H,10-20H2,1-3H3,(H2,34,41)(H,36,42)/t22?,23?,24?,29-,33- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433412
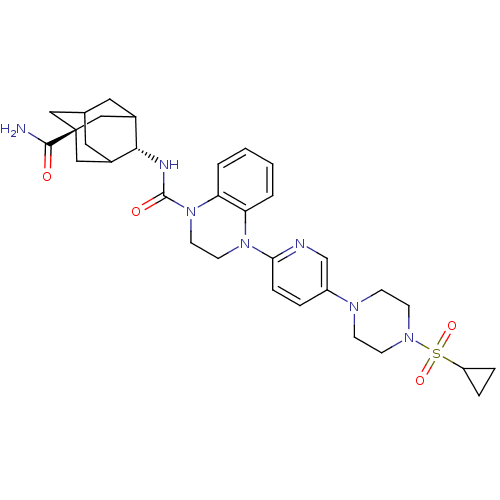
(CHEMBL2380649)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)N1CCN(c4ccc(cn4)N4CCN(CC4)S(=O)(=O)C4CC4)c4ccccc14)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:1:3:9.41.42:6,10:9:3.8.43:42.5.6,10:9:6:3.8.4,THB:43:3:9.41.42:6,43:41:6:3.8.4,4:3:9:42.5.6,4:5:9:3.8.43,(26.96,-60.09,;26.19,-58.76,;26.96,-57.43,;24.65,-58.76,;24.66,-57.23,;23.27,-56.65,;21.92,-57.13,;21.93,-58.62,;23.26,-59.11,;20.73,-59.89,;19.19,-59.87,;18.27,-61.1,;19.04,-62.44,;16.73,-61.1,;15.96,-62.43,;14.42,-62.43,;13.65,-61.09,;12.11,-61.09,;11.33,-59.75,;9.8,-59.75,;9.03,-61.08,;9.8,-62.42,;11.34,-62.42,;7.5,-61.09,;6.73,-62.42,;5.2,-62.41,;4.43,-61.09,;5.19,-59.76,;6.73,-59.76,;2.89,-61.09,;2.88,-62.63,;1.55,-61.85,;2.12,-59.77,;2.1,-58.24,;.78,-59.01,;14.42,-59.75,;13.65,-58.42,;14.43,-57.08,;15.98,-57.09,;16.75,-58.43,;15.97,-59.77,;22.23,-59.47,;22.23,-57.89,;23.64,-60.04,)| Show InChI InChI=1S/C32H41N7O4S/c33-30(40)32-17-21-15-22(18-32)29(23(16-21)19-32)35-31(41)39-14-13-38(26-3-1-2-4-27(26)39)28-8-5-24(20-34-28)36-9-11-37(12-10-36)44(42,43)25-6-7-25/h1-5,8,20-23,25,29H,6-7,9-19H2,(H2,33,40)(H,35,41)/t21?,22?,23?,29-,32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340438
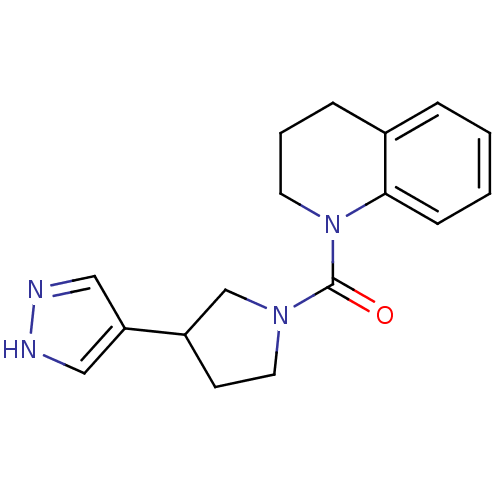
((3-(1H-pyrazol-4-yl)pyrrolidin-1-yl)(3,4-dihydroqu...)Show InChI InChI=1S/C17H20N4O/c22-17(20-9-7-14(12-20)15-10-18-19-11-15)21-8-3-5-13-4-1-2-6-16(13)21/h1-2,4,6,10-11,14H,3,5,7-9,12H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 assessed as conversion of radiolabelled-cortisone to cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438083
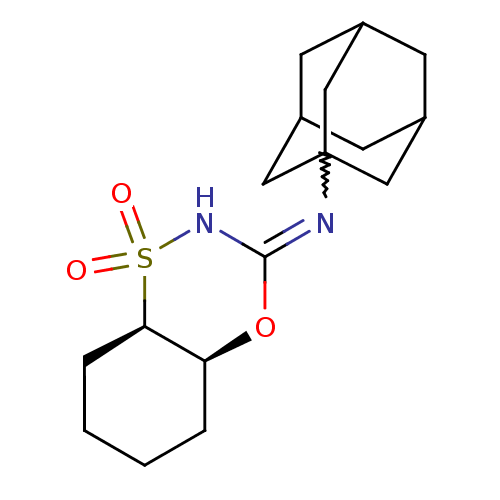
(CHEMBL2409755)Show SMILES O=S1(=O)NC(O[C@H]2CCCC[C@@H]12)=NC12CC3CC(CC(C3)C1)C2 |r,w:12.14,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,TEB:12:13:16:20.19.18,12:13:16.15.20:18| Show InChI InChI=1S/C17H26N2O3S/c20-23(21)15-4-2-1-3-14(15)22-16(19-23)18-17-8-11-5-12(9-17)7-13(6-11)10-17/h11-15H,1-10H2,(H,18,19)/t11?,12?,13?,14-,15+,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438070
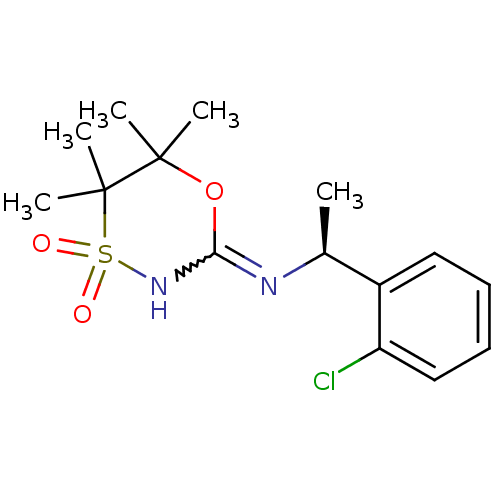
(CHEMBL2409724)Show SMILES C[C@H](N=C1NS(=O)(=O)C(C)(C)C(C)(C)O1)c1ccccc1Cl |r,w:3.3| Show InChI InChI=1S/C15H21ClN2O3S/c1-10(11-8-6-7-9-12(11)16)17-13-18-22(19,20)15(4,5)14(2,3)21-13/h6-10H,1-5H3,(H,17,18)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438080
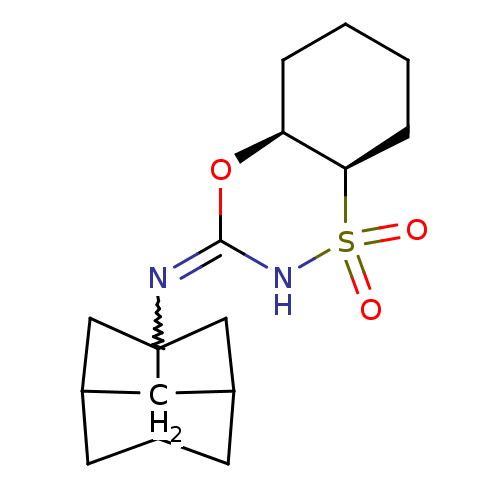
(CHEMBL2409607)Show SMILES O=S1(=O)NC(O[C@H]2CCCC[C@@H]12)=NC12CC3CC1CC(C2)C3 |r,w:12.14,TLB:20:19:16:13.14,THB:20:13:16:19.18.21,TEB:12:13:16:19.18.21,12:13:18:16.15.21| Show InChI InChI=1S/C16H24N2O3S/c19-22(20)14-4-2-1-3-13(14)21-15(18-22)17-16-8-10-5-11(9-16)7-12(16)6-10/h10-14H,1-9H2,(H,17,18)/t10?,11?,12?,13-,14+,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438068
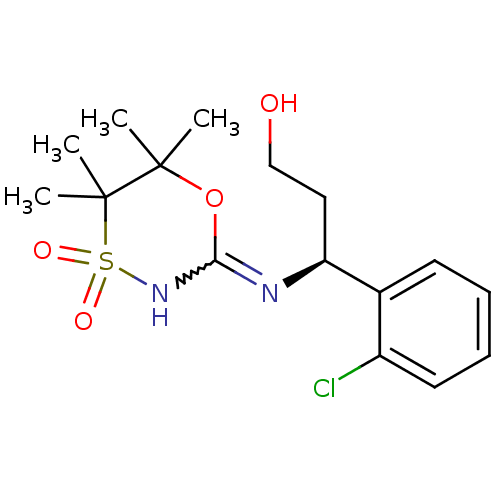
(CHEMBL2409726)Show SMILES CC1(C)OC(NS(=O)(=O)C1(C)C)=N[C@@H](CCO)c1ccccc1Cl |r,w:4.4| Show InChI InChI=1S/C16H23ClN2O4S/c1-15(2)16(3,4)24(21,22)19-14(23-15)18-13(9-10-20)11-7-5-6-8-12(11)17/h5-8,13,20H,9-10H2,1-4H3,(H,18,19)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433400
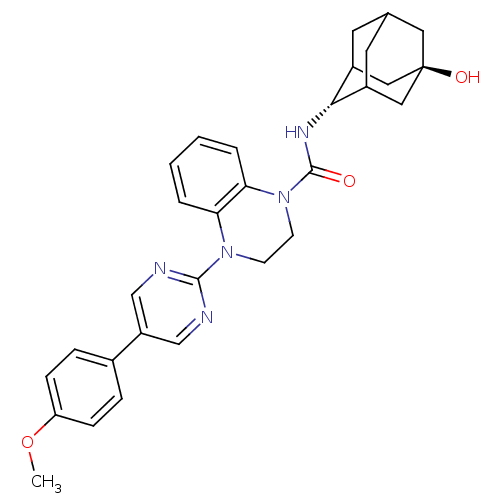
(CHEMBL2380637)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:21.22,wD:28.31,TLB:20:21:31.27.28:25.24.23,20:21:23:31.28.30,29:28:21.26.25:23,THB:27:26:23:31.28.30,27:28:21.26.25:23,29:28:21:25.24.23,30:28:21:25.24.23,30:24:21:31.27.28,(.2,-12.39,;.98,-13.73,;2.52,-13.72,;3.29,-15.05,;4.83,-15.05,;5.59,-13.72,;4.83,-12.39,;3.28,-12.39,;7.13,-13.71,;7.9,-15.05,;9.44,-15.05,;10.21,-13.72,;9.43,-12.38,;7.9,-12.38,;11.76,-13.72,;12.53,-15.06,;14.06,-15.06,;14.84,-13.73,;16.38,-13.73,;17.15,-15.07,;17.3,-12.49,;18.84,-12.52,;20.04,-11.24,;20.03,-9.76,;21.38,-9.28,;20.34,-10.51,;20.35,-12.1,;21.75,-12.67,;22.77,-11.39,;24.31,-11.33,;22.78,-9.86,;21.37,-11.73,;14.07,-12.39,;14.85,-11.06,;14.09,-9.71,;12.53,-9.71,;11.76,-11.04,;12.52,-12.38,)| Show InChI InChI=1S/C30H33N5O3/c1-38-24-8-6-20(7-9-24)23-17-31-28(32-18-23)34-10-11-35(26-5-3-2-4-25(26)34)29(36)33-27-21-12-19-13-22(27)16-30(37,14-19)15-21/h2-9,17-19,21-22,27,37H,10-16H2,1H3,(H,33,36)/t19?,21?,22?,27-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433407
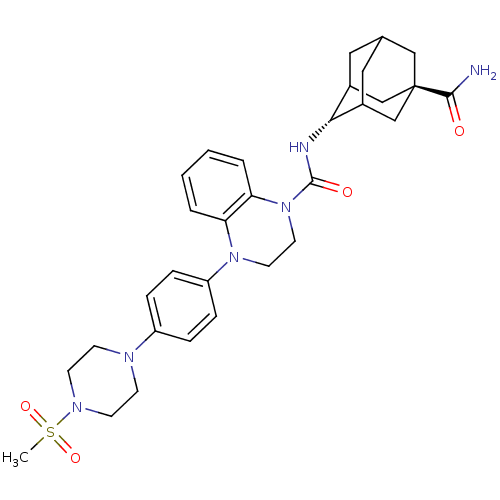
(CHEMBL2380644)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:23.24,wD:30.37,TLB:33:30:23.28.27:25,22:23:30.32.29:27.26.25,22:23:25:30.32.31,THB:29:30:23.28.27:25,29:28:25:30.32.31,31:30:23:27.26.25,31:26:23:30.32.29,(28.99,-37.06,;29.76,-38.38,;29.75,-39.92,;28.42,-39.15,;31.3,-38.39,;32.07,-39.71,;33.6,-39.71,;34.37,-38.38,;33.6,-37.05,;32.06,-37.06,;35.9,-38.38,;36.67,-39.72,;38.21,-39.72,;38.98,-38.38,;38.21,-37.04,;36.67,-37.05,;40.52,-38.38,;41.29,-39.72,;42.83,-39.72,;43.6,-38.39,;45.14,-38.4,;45.91,-39.73,;46.06,-37.16,;47.59,-37.19,;48.79,-35.92,;48.78,-34.43,;50.13,-33.95,;49.09,-35.19,;49.1,-36.77,;50.5,-37.34,;51.51,-36.06,;51.53,-34.53,;50.12,-36.41,;53.05,-36.06,;53.82,-34.73,;53.82,-37.39,;42.84,-37.06,;43.62,-35.73,;42.85,-34.38,;41.3,-34.38,;40.52,-35.71,;41.29,-37.05,)| Show InChI InChI=1S/C31H40N6O4S/c1-42(40,41)35-12-10-34(11-13-35)24-6-8-25(9-7-24)36-14-15-37(27-5-3-2-4-26(27)36)30(39)33-28-22-16-21-17-23(28)20-31(18-21,19-22)29(32)38/h2-9,21-23,28H,10-20H2,1H3,(H2,32,38)(H,33,39)/t21?,22?,23?,28-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433403
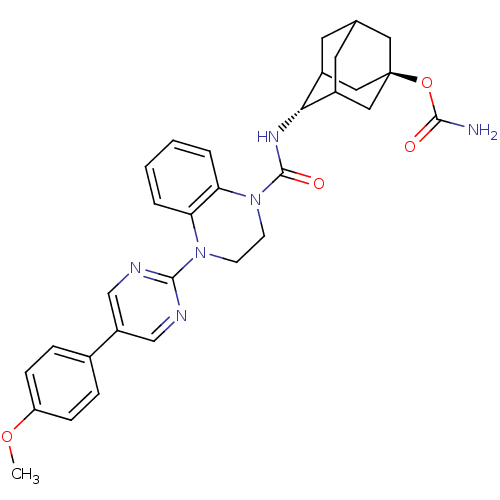
(CHEMBL2380640)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)OC(N)=O)c2ccccc12 |r,wU:21.22,wD:28.35,TLB:31:28:21.26.25:23,20:21:28.30.27:25.24.23,20:21:23:28.30.29,THB:27:28:21.26.25:23,27:26:23:28.30.29,29:28:21:25.24.23,29:24:21:28.30.27,(27.37,-22.08,;28.15,-23.41,;29.69,-23.41,;30.45,-24.73,;31.99,-24.73,;32.76,-23.4,;31.99,-22.07,;30.45,-22.08,;34.29,-23.4,;35.06,-24.74,;36.6,-24.74,;37.37,-23.4,;36.59,-22.06,;35.06,-22.07,;38.91,-23.4,;39.68,-24.74,;41.22,-24.74,;41.99,-23.41,;43.53,-23.42,;44.3,-24.75,;44.45,-22.18,;45.99,-22.21,;47.19,-20.93,;47.18,-19.45,;48.53,-18.97,;47.49,-20.2,;47.49,-21.79,;48.9,-22.35,;49.91,-21.08,;49.92,-19.55,;48.52,-21.42,;51.45,-21.08,;52.22,-22.41,;53.76,-22.41,;51.45,-23.74,;41.22,-22.08,;42.01,-20.75,;41.24,-19.4,;39.68,-19.4,;38.91,-20.73,;39.68,-22.07,)| Show InChI InChI=1S/C31H34N6O4/c1-40-24-8-6-20(7-9-24)23-17-33-29(34-18-23)36-10-11-37(26-5-3-2-4-25(26)36)30(39)35-27-21-12-19-13-22(27)16-31(14-19,15-21)41-28(32)38/h2-9,17-19,21-22,27H,10-16H2,1H3,(H2,32,38)(H,35,39)/t19?,21?,22?,27-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433407

(CHEMBL2380644)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:23.24,wD:30.37,TLB:33:30:23.28.27:25,22:23:30.32.29:27.26.25,22:23:25:30.32.31,THB:29:30:23.28.27:25,29:28:25:30.32.31,31:30:23:27.26.25,31:26:23:30.32.29,(28.99,-37.06,;29.76,-38.38,;29.75,-39.92,;28.42,-39.15,;31.3,-38.39,;32.07,-39.71,;33.6,-39.71,;34.37,-38.38,;33.6,-37.05,;32.06,-37.06,;35.9,-38.38,;36.67,-39.72,;38.21,-39.72,;38.98,-38.38,;38.21,-37.04,;36.67,-37.05,;40.52,-38.38,;41.29,-39.72,;42.83,-39.72,;43.6,-38.39,;45.14,-38.4,;45.91,-39.73,;46.06,-37.16,;47.59,-37.19,;48.79,-35.92,;48.78,-34.43,;50.13,-33.95,;49.09,-35.19,;49.1,-36.77,;50.5,-37.34,;51.51,-36.06,;51.53,-34.53,;50.12,-36.41,;53.05,-36.06,;53.82,-34.73,;53.82,-37.39,;42.84,-37.06,;43.62,-35.73,;42.85,-34.38,;41.3,-34.38,;40.52,-35.71,;41.29,-37.05,)| Show InChI InChI=1S/C31H40N6O4S/c1-42(40,41)35-12-10-34(11-13-35)24-6-8-25(9-7-24)36-14-15-37(27-5-3-2-4-26(27)36)30(39)33-28-22-16-21-17-23(28)20-31(18-21,19-22)29(32)38/h2-9,21-23,28H,10-20H2,1H3,(H2,32,38)(H,33,39)/t21?,22?,23?,28-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438067
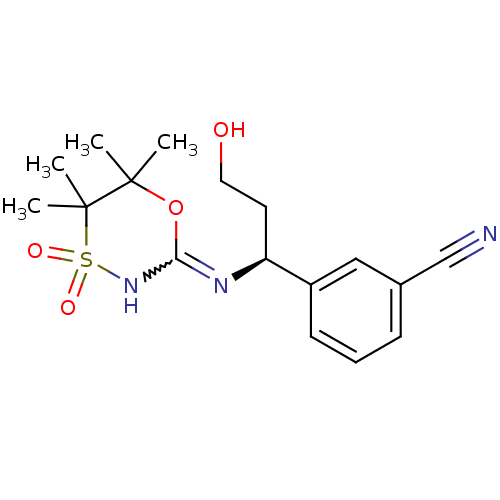
(CHEMBL2409727)Show SMILES CC1(C)OC(NS(=O)(=O)C1(C)C)=N[C@@H](CCO)c1cccc(c1)C#N |r,w:4.4| Show InChI InChI=1S/C17H23N3O4S/c1-16(2)17(3,4)25(22,23)20-15(24-16)19-14(8-9-21)13-7-5-6-12(10-13)11-18/h5-7,10,14,21H,8-9H2,1-4H3,(H,19,20)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433411

(CHEMBL2380648)Show SMILES CC(C)(C)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:23.24,wD:30.37,TLB:33:30:23.28.27:25,22:23:30.32.29:27.26.25,22:23:25:30.32.31,THB:29:30:23.28.27:25,29:28:25:30.32.31,31:30:23:27.26.25,31:26:23:30.32.29,(27.21,-52.3,;27.98,-53.63,;27.21,-54.97,;26.44,-53.63,;29.52,-53.63,;30.29,-54.95,;31.82,-54.95,;32.59,-53.63,;31.82,-52.3,;30.28,-52.3,;34.12,-53.62,;34.89,-52.29,;36.42,-52.29,;37.2,-53.63,;36.43,-54.96,;34.89,-54.96,;38.74,-53.62,;39.51,-54.96,;41.04,-54.97,;41.82,-53.63,;43.36,-53.64,;44.13,-54.97,;44.28,-52.41,;45.81,-52.44,;47.01,-51.16,;47,-49.68,;48.35,-49.2,;47.31,-50.43,;47.31,-52.02,;48.72,-52.58,;49.73,-51.3,;49.74,-49.78,;48.34,-51.65,;51.27,-51.3,;52.04,-52.64,;52.04,-49.97,;41.05,-52.3,;41.83,-50.97,;41.07,-49.63,;39.51,-49.62,;38.74,-50.96,;39.51,-52.29,)| Show InChI InChI=1S/C33H45N7O2/c1-32(2,3)38-12-10-37(11-13-38)25-8-9-28(35-21-25)39-14-15-40(27-7-5-4-6-26(27)39)31(42)36-29-23-16-22-17-24(29)20-33(18-22,19-23)30(34)41/h4-9,21-24,29H,10-20H2,1-3H3,(H2,34,41)(H,36,42)/t22?,23?,24?,29-,33- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50433411

(CHEMBL2380648)Show SMILES CC(C)(C)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:23.24,wD:30.37,TLB:33:30:23.28.27:25,22:23:30.32.29:27.26.25,22:23:25:30.32.31,THB:29:30:23.28.27:25,29:28:25:30.32.31,31:30:23:27.26.25,31:26:23:30.32.29,(27.21,-52.3,;27.98,-53.63,;27.21,-54.97,;26.44,-53.63,;29.52,-53.63,;30.29,-54.95,;31.82,-54.95,;32.59,-53.63,;31.82,-52.3,;30.28,-52.3,;34.12,-53.62,;34.89,-52.29,;36.42,-52.29,;37.2,-53.63,;36.43,-54.96,;34.89,-54.96,;38.74,-53.62,;39.51,-54.96,;41.04,-54.97,;41.82,-53.63,;43.36,-53.64,;44.13,-54.97,;44.28,-52.41,;45.81,-52.44,;47.01,-51.16,;47,-49.68,;48.35,-49.2,;47.31,-50.43,;47.31,-52.02,;48.72,-52.58,;49.73,-51.3,;49.74,-49.78,;48.34,-51.65,;51.27,-51.3,;52.04,-52.64,;52.04,-49.97,;41.05,-52.3,;41.83,-50.97,;41.07,-49.63,;39.51,-49.62,;38.74,-50.96,;39.51,-52.29,)| Show InChI InChI=1S/C33H45N7O2/c1-32(2,3)38-12-10-37(11-13-38)25-8-9-28(35-21-25)39-14-15-40(27-7-5-4-6-26(27)39)31(42)36-29-23-16-22-17-24(29)20-33(18-22,19-23)30(34)41/h4-9,21-24,29H,10-20H2,1-3H3,(H2,34,41)(H,36,42)/t22?,23?,24?,29-,33- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50340439

((3-(1H-pyrazol-4-yl)pyrrolidin-1-yl)(4,4-dimethyl-...)Show SMILES CC1(C)CCN(C(=O)N2CCC(C2)c2cn[nH]c2)c2ccccc12 Show InChI InChI=1S/C19H24N4O/c1-19(2)8-10-23(17-6-4-3-5-16(17)19)18(24)22-9-7-14(13-22)15-11-20-21-12-15/h3-6,11-12,14H,7-10,13H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 assessed as conversion of radiolabelled-cortisone to cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438069
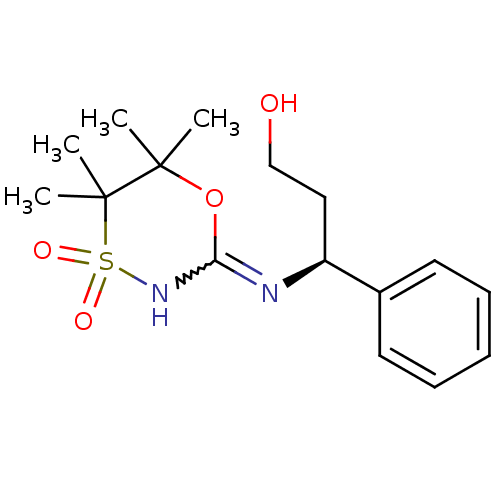
(CHEMBL2409725)Show SMILES CC1(C)OC(NS(=O)(=O)C1(C)C)=N[C@@H](CCO)c1ccccc1 |r,w:4.4| Show InChI InChI=1S/C16H24N2O4S/c1-15(2)16(3,4)23(20,21)18-14(22-15)17-13(10-11-19)12-8-6-5-7-9-12/h5-9,13,19H,10-11H2,1-4H3,(H,17,18)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438066
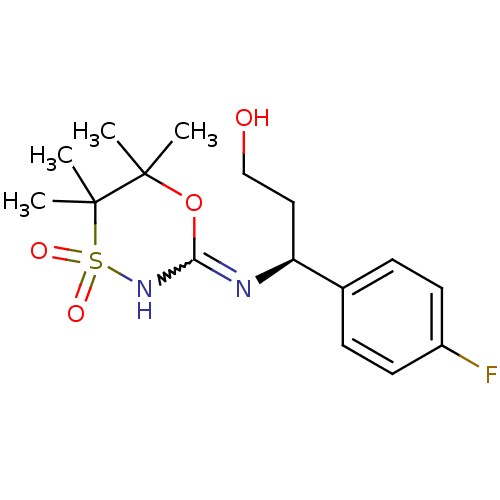
(CHEMBL2409728)Show SMILES CC1(C)OC(NS(=O)(=O)C1(C)C)=N[C@@H](CCO)c1ccc(F)cc1 |r,w:4.4| Show InChI InChI=1S/C16H23FN2O4S/c1-15(2)16(3,4)24(21,22)19-14(23-15)18-13(9-10-20)11-5-7-12(17)8-6-11/h5-8,13,20H,9-10H2,1-4H3,(H,18,19)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340442

((3-(1H-pyrazol-4-yl)pyrrolidin-1-yl)(3,4-dihydroqu...)Show InChI InChI=1S/C16H19N5O/c22-16(20-7-5-12(11-20)13-9-18-19-10-13)21-8-6-17-14-3-1-2-4-15(14)21/h1-4,9-10,12,17H,5-8,11H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 assessed as conversion of radiolabelled-cortisone to cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433408
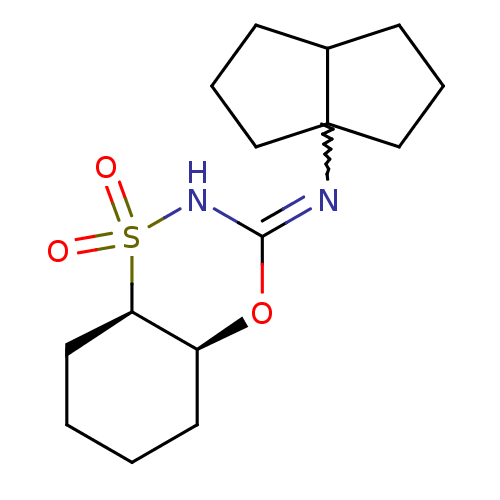
(CHEMBL2380645)Show SMILES CC(C)(C)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(1.87,-44.23,;2.65,-45.56,;1.88,-46.9,;1.1,-45.55,;4.19,-45.56,;4.95,-46.88,;6.49,-46.88,;7.26,-45.55,;6.49,-44.22,;4.95,-44.23,;8.79,-45.55,;9.56,-44.22,;11.09,-44.21,;11.87,-45.55,;11.1,-46.89,;9.56,-46.89,;13.41,-45.55,;14.18,-46.89,;15.71,-46.89,;16.49,-45.56,;18.03,-45.57,;18.8,-46.9,;18.95,-44.33,;20.49,-44.36,;21.68,-43.08,;21.67,-41.6,;23.02,-41.12,;21.98,-42.35,;21.99,-43.94,;23.39,-44.5,;24.41,-43.23,;25.95,-43.23,;24.42,-41.7,;23.01,-43.57,;15.72,-44.23,;16.5,-42.9,;15.74,-41.55,;14.18,-41.55,;13.41,-42.88,;14.17,-44.22,)| Show InChI InChI=1S/C32H44N6O2/c1-31(2,3)36-12-10-35(11-13-36)25-8-9-28(33-21-25)37-14-15-38(27-7-5-4-6-26(27)37)30(39)34-29-23-16-22-17-24(29)20-32(40,18-22)19-23/h4-9,21-24,29,40H,10-20H2,1-3H3,(H,34,39)/t22?,23?,24?,29-,32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438087
(CHEMBL2409751)Show SMILES O=S1(=O)NC(O[C@H]2CCCC[C@@H]12)=NC12CCCC1CCC2 |r,w:12.14| Show InChI InChI=1S/C15H24N2O3S/c18-21(19)13-8-2-1-7-12(13)20-14(17-21)16-15-9-3-5-11(15)6-4-10-15/h11-13H,1-10H2,(H,16,17)/t11?,12-,13+,15?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340439

((3-(1H-pyrazol-4-yl)pyrrolidin-1-yl)(4,4-dimethyl-...)Show SMILES CC1(C)CCN(C(=O)N2CCC(C2)c2cn[nH]c2)c2ccccc12 Show InChI InChI=1S/C19H24N4O/c1-19(2)8-10-23(17-6-4-3-5-16(17)19)18(24)22-9-7-14(13-22)15-11-20-21-12-15/h3-6,11-12,14H,7-10,13H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 assessed as conversion of radiolabelled-cortisone to cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433412

(CHEMBL2380649)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)N1CCN(c4ccc(cn4)N4CCN(CC4)S(=O)(=O)C4CC4)c4ccccc14)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:1:3:9.41.42:6,10:9:3.8.43:42.5.6,10:9:6:3.8.4,THB:43:3:9.41.42:6,43:41:6:3.8.4,4:3:9:42.5.6,4:5:9:3.8.43,(26.96,-60.09,;26.19,-58.76,;26.96,-57.43,;24.65,-58.76,;24.66,-57.23,;23.27,-56.65,;21.92,-57.13,;21.93,-58.62,;23.26,-59.11,;20.73,-59.89,;19.19,-59.87,;18.27,-61.1,;19.04,-62.44,;16.73,-61.1,;15.96,-62.43,;14.42,-62.43,;13.65,-61.09,;12.11,-61.09,;11.33,-59.75,;9.8,-59.75,;9.03,-61.08,;9.8,-62.42,;11.34,-62.42,;7.5,-61.09,;6.73,-62.42,;5.2,-62.41,;4.43,-61.09,;5.19,-59.76,;6.73,-59.76,;2.89,-61.09,;2.88,-62.63,;1.55,-61.85,;2.12,-59.77,;2.1,-58.24,;.78,-59.01,;14.42,-59.75,;13.65,-58.42,;14.43,-57.08,;15.98,-57.09,;16.75,-58.43,;15.97,-59.77,;22.23,-59.47,;22.23,-57.89,;23.64,-60.04,)| Show InChI InChI=1S/C32H41N7O4S/c33-30(40)32-17-21-15-22(18-32)29(23(16-21)19-32)35-31(41)39-14-13-38(26-3-1-2-4-27(26)39)28-8-5-24(20-34-28)36-9-11-37(12-10-36)44(42,43)25-6-7-25/h1-5,8,20-23,25,29H,6-7,9-19H2,(H2,33,40)(H,35,41)/t21?,22?,23?,29-,32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433403

(CHEMBL2380640)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)OC(N)=O)c2ccccc12 |r,wU:21.22,wD:28.35,TLB:31:28:21.26.25:23,20:21:28.30.27:25.24.23,20:21:23:28.30.29,THB:27:28:21.26.25:23,27:26:23:28.30.29,29:28:21:25.24.23,29:24:21:28.30.27,(27.37,-22.08,;28.15,-23.41,;29.69,-23.41,;30.45,-24.73,;31.99,-24.73,;32.76,-23.4,;31.99,-22.07,;30.45,-22.08,;34.29,-23.4,;35.06,-24.74,;36.6,-24.74,;37.37,-23.4,;36.59,-22.06,;35.06,-22.07,;38.91,-23.4,;39.68,-24.74,;41.22,-24.74,;41.99,-23.41,;43.53,-23.42,;44.3,-24.75,;44.45,-22.18,;45.99,-22.21,;47.19,-20.93,;47.18,-19.45,;48.53,-18.97,;47.49,-20.2,;47.49,-21.79,;48.9,-22.35,;49.91,-21.08,;49.92,-19.55,;48.52,-21.42,;51.45,-21.08,;52.22,-22.41,;53.76,-22.41,;51.45,-23.74,;41.22,-22.08,;42.01,-20.75,;41.24,-19.4,;39.68,-19.4,;38.91,-20.73,;39.68,-22.07,)| Show InChI InChI=1S/C31H34N6O4/c1-40-24-8-6-20(7-9-24)23-17-33-29(34-18-23)36-10-11-37(26-5-3-2-4-25(26)36)30(39)35-27-21-12-19-13-22(27)16-31(14-19,15-21)41-28(32)38/h2-9,17-19,21-22,27H,10-16H2,1H3,(H2,32,38)(H,35,39)/t19?,21?,22?,27-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433408

(CHEMBL2380645)Show SMILES CC(C)(C)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(1.87,-44.23,;2.65,-45.56,;1.88,-46.9,;1.1,-45.55,;4.19,-45.56,;4.95,-46.88,;6.49,-46.88,;7.26,-45.55,;6.49,-44.22,;4.95,-44.23,;8.79,-45.55,;9.56,-44.22,;11.09,-44.21,;11.87,-45.55,;11.1,-46.89,;9.56,-46.89,;13.41,-45.55,;14.18,-46.89,;15.71,-46.89,;16.49,-45.56,;18.03,-45.57,;18.8,-46.9,;18.95,-44.33,;20.49,-44.36,;21.68,-43.08,;21.67,-41.6,;23.02,-41.12,;21.98,-42.35,;21.99,-43.94,;23.39,-44.5,;24.41,-43.23,;25.95,-43.23,;24.42,-41.7,;23.01,-43.57,;15.72,-44.23,;16.5,-42.9,;15.74,-41.55,;14.18,-41.55,;13.41,-42.88,;14.17,-44.22,)| Show InChI InChI=1S/C32H44N6O2/c1-31(2,3)36-12-10-35(11-13-36)25-8-9-28(33-21-25)37-14-15-38(27-7-5-4-6-26(27)37)30(39)34-29-23-16-22-17-24(29)20-32(40,18-22)19-23/h4-9,21-24,29,40H,10-20H2,1-3H3,(H,34,39)/t22?,23?,24?,29-,32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50438080

(CHEMBL2409607)Show SMILES O=S1(=O)NC(O[C@H]2CCCC[C@@H]12)=NC12CC3CC1CC(C2)C3 |r,w:12.14,TLB:20:19:16:13.14,THB:20:13:16:19.18.21,TEB:12:13:16:19.18.21,12:13:18:16.15.21| Show InChI InChI=1S/C16H24N2O3S/c19-22(20)14-4-2-1-3-13(14)21-15(18-22)17-16-8-10-5-11(9-16)7-12(16)6-10/h10-14H,1-9H2,(H,17,18)/t10?,11?,12?,13-,14+,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50433412

(CHEMBL2380649)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)N1CCN(c4ccc(cn4)N4CCN(CC4)S(=O)(=O)C4CC4)c4ccccc14)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:1:3:9.41.42:6,10:9:3.8.43:42.5.6,10:9:6:3.8.4,THB:43:3:9.41.42:6,43:41:6:3.8.4,4:3:9:42.5.6,4:5:9:3.8.43,(26.96,-60.09,;26.19,-58.76,;26.96,-57.43,;24.65,-58.76,;24.66,-57.23,;23.27,-56.65,;21.92,-57.13,;21.93,-58.62,;23.26,-59.11,;20.73,-59.89,;19.19,-59.87,;18.27,-61.1,;19.04,-62.44,;16.73,-61.1,;15.96,-62.43,;14.42,-62.43,;13.65,-61.09,;12.11,-61.09,;11.33,-59.75,;9.8,-59.75,;9.03,-61.08,;9.8,-62.42,;11.34,-62.42,;7.5,-61.09,;6.73,-62.42,;5.2,-62.41,;4.43,-61.09,;5.19,-59.76,;6.73,-59.76,;2.89,-61.09,;2.88,-62.63,;1.55,-61.85,;2.12,-59.77,;2.1,-58.24,;.78,-59.01,;14.42,-59.75,;13.65,-58.42,;14.43,-57.08,;15.98,-57.09,;16.75,-58.43,;15.97,-59.77,;22.23,-59.47,;22.23,-57.89,;23.64,-60.04,)| Show InChI InChI=1S/C32H41N7O4S/c33-30(40)32-17-21-15-22(18-32)29(23(16-21)19-32)35-31(41)39-14-13-38(26-3-1-2-4-27(26)39)28-8-5-24(20-34-28)36-9-11-37(12-10-36)44(42,43)25-6-7-25/h1-5,8,20-23,25,29H,6-7,9-19H2,(H2,33,40)(H,35,41)/t21?,22?,23?,29-,32- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340443

((3-(1H-pyrazol-4-yl)pyrrolidin-1-yl)(4-methyl-3,4-...)Show InChI InChI=1S/C17H21N5O/c1-20-8-9-22(16-5-3-2-4-15(16)20)17(23)21-7-6-13(12-21)14-10-18-19-11-14/h2-5,10-11,13H,6-9,12H2,1H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 assessed as conversion of radiolabelled-cortisone to cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433410
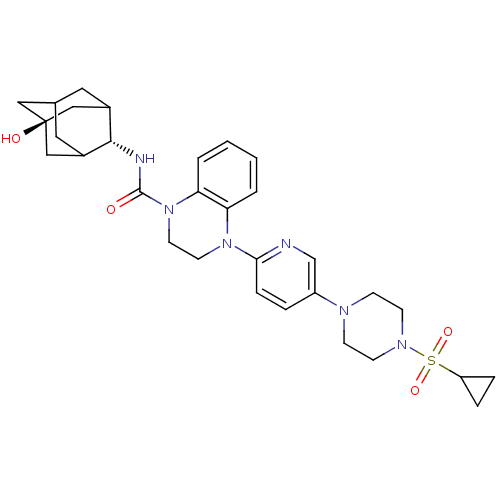
(CHEMBL2380647)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)N1CCN(c4ccc(cn4)N4CCN(CC4)S(=O)(=O)C4CC4)c4ccccc14)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7.39.40:4,8:7:1.6.41:40.3.4,8:7:4:1.6.2,THB:41:1:7.39.40:4,41:39:4:1.6.2,2:1:7:40.3.4,2:3:7:1.6.41,(26.68,-51.08,;25.14,-51.08,;25.15,-49.56,;23.75,-48.98,;22.41,-49.46,;22.41,-50.94,;23.74,-51.43,;21.22,-52.22,;19.68,-52.19,;18.76,-53.43,;19.53,-54.76,;17.22,-53.42,;16.44,-54.75,;14.91,-54.75,;14.14,-53.41,;12.6,-53.41,;11.82,-52.07,;10.28,-52.08,;9.52,-53.41,;10.29,-54.75,;11.83,-54.75,;7.98,-53.41,;7.22,-54.74,;5.68,-54.74,;4.91,-53.42,;5.67,-52.09,;7.22,-52.08,;3.37,-53.41,;3.36,-54.95,;2.03,-54.18,;2.6,-52.09,;2.59,-50.56,;1.26,-51.34,;14.9,-52.08,;14.14,-50.74,;14.91,-49.41,;16.47,-49.41,;17.23,-50.76,;16.45,-52.09,;22.72,-51.8,;22.71,-50.21,;24.12,-52.36,)| Show InChI InChI=1S/C31H40N6O4S/c38-30(33-29-22-15-21-16-23(29)19-31(39,17-21)18-22)37-14-13-36(26-3-1-2-4-27(26)37)28-8-5-24(20-32-28)34-9-11-35(12-10-34)42(40,41)25-6-7-25/h1-5,8,20-23,25,29,39H,6-7,9-19H2,(H,33,38)/t21?,22?,23?,29-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50340432
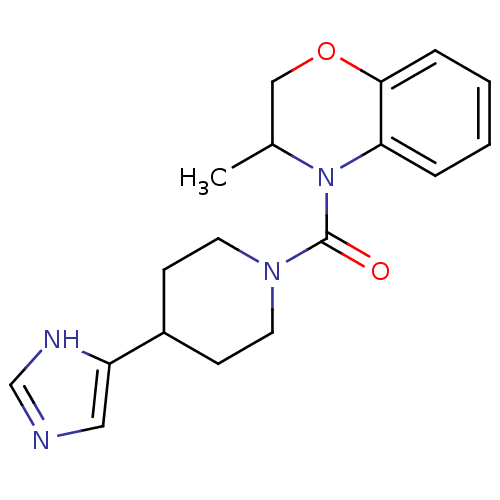
((4-(1H-imidazol-4-yl)piperidin-1-yl)(3-methyl-2H-b...)Show InChI InChI=1S/C18H22N4O2/c1-13-11-24-17-5-3-2-4-16(17)22(13)18(23)21-8-6-14(7-9-21)15-10-19-12-20-15/h2-5,10,12-14H,6-9,11H2,1H3,(H,19,20) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD2 |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438065
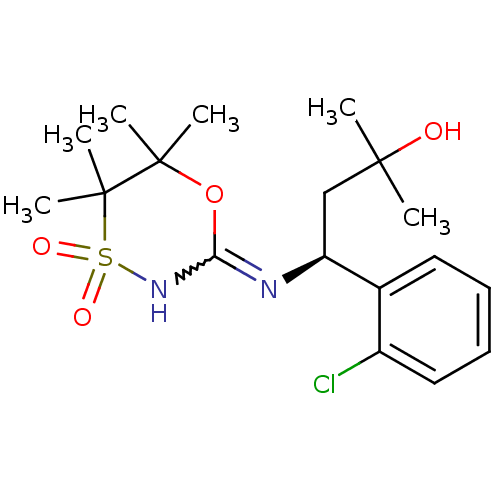
(CHEMBL2409729)Show SMILES CC(C)(O)C[C@H](N=C1NS(=O)(=O)C(C)(C)C(C)(C)O1)c1ccccc1Cl |r,w:7.7| Show InChI InChI=1S/C18H27ClN2O4S/c1-16(2,22)11-14(12-9-7-8-10-13(12)19)20-15-21-26(23,24)18(5,6)17(3,4)25-15/h7-10,14,22H,11H2,1-6H3,(H,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50340431
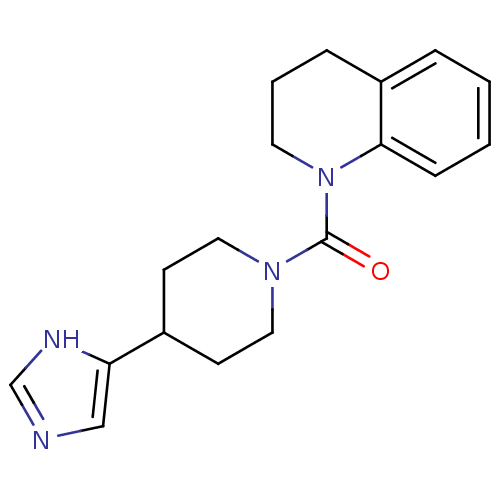
((4-(1H-imidazol-4-yl)piperidin-1-yl)(3,4-dihydroqu...)Show InChI InChI=1S/C18H22N4O/c23-18(22-9-3-5-15-4-1-2-6-17(15)22)21-10-7-14(8-11-21)16-12-19-13-20-16/h1-2,4,6,12-14H,3,5,7-11H2,(H,19,20) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD2 |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433409
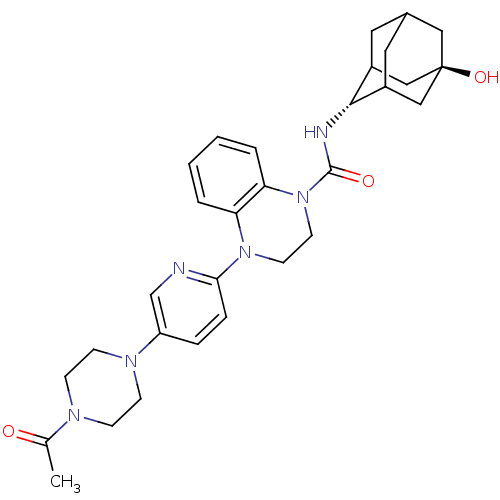
(CHEMBL2380646)Show SMILES CC(=O)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:22.23,wD:29.32,TLB:30:29:22.27.26:24,21:22:29.32.28:26.25.24,21:22:24:29.32.31,THB:28:29:22.27.26:24,28:27:24:29.32.31,31:29:22:26.25.24,31:25:22:29.32.28,(27.96,-44.51,;28.73,-45.83,;27.96,-47.17,;30.28,-45.84,;31.04,-47.16,;32.58,-47.16,;33.35,-45.83,;32.58,-44.5,;31.04,-44.51,;34.88,-45.83,;35.65,-44.5,;37.18,-44.49,;37.96,-45.83,;37.19,-47.17,;35.65,-47.17,;39.5,-45.83,;40.27,-47.17,;41.81,-47.17,;42.58,-45.84,;44.12,-45.85,;44.89,-47.18,;45.04,-44.61,;46.58,-44.64,;47.78,-43.36,;47.77,-41.88,;49.12,-41.4,;48.08,-42.63,;48.08,-44.22,;49.49,-44.78,;50.5,-43.51,;52.04,-43.51,;50.51,-41.98,;49.11,-43.85,;41.82,-44.51,;42.6,-43.18,;41.83,-41.83,;40.28,-41.83,;39.5,-43.16,;40.27,-44.5,)| Show InChI InChI=1S/C30H38N6O3/c1-20(37)33-8-10-34(11-9-33)24-6-7-27(31-19-24)35-12-13-36(26-5-3-2-4-25(26)35)29(38)32-28-22-14-21-15-23(28)18-30(39,16-21)17-22/h2-7,19,21-23,28,39H,8-18H2,1H3,(H,32,38)/t21?,22?,23?,28-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438090

(CHEMBL2409747)Show SMILES CC1(C)CC(CC(C)(C)C1)N=C1NS(=O)(=O)[C@@H]2CCCC[C@@H]2O1 |r,w:10.10| Show InChI InChI=1S/C17H30N2O3S/c1-16(2)9-12(10-17(3,4)11-16)18-15-19-23(20,21)14-8-6-5-7-13(14)22-15/h12-14H,5-11H2,1-4H3,(H,18,19)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438077
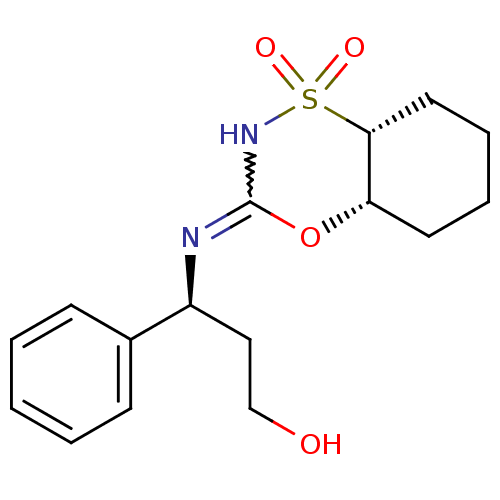
(CHEMBL2409612)Show SMILES OCC[C@H](N=C1NS(=O)(=O)[C@@H]2CCCC[C@@H]2O1)c1ccccc1 |r,w:5.5| Show InChI InChI=1S/C16H22N2O4S/c19-11-10-13(12-6-2-1-3-7-12)17-16-18-23(20,21)15-9-5-4-8-14(15)22-16/h1-3,6-7,13-15,19H,4-5,8-11H2,(H,17,18)/t13-,14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433409

(CHEMBL2380646)Show SMILES CC(=O)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:22.23,wD:29.32,TLB:30:29:22.27.26:24,21:22:29.32.28:26.25.24,21:22:24:29.32.31,THB:28:29:22.27.26:24,28:27:24:29.32.31,31:29:22:26.25.24,31:25:22:29.32.28,(27.96,-44.51,;28.73,-45.83,;27.96,-47.17,;30.28,-45.84,;31.04,-47.16,;32.58,-47.16,;33.35,-45.83,;32.58,-44.5,;31.04,-44.51,;34.88,-45.83,;35.65,-44.5,;37.18,-44.49,;37.96,-45.83,;37.19,-47.17,;35.65,-47.17,;39.5,-45.83,;40.27,-47.17,;41.81,-47.17,;42.58,-45.84,;44.12,-45.85,;44.89,-47.18,;45.04,-44.61,;46.58,-44.64,;47.78,-43.36,;47.77,-41.88,;49.12,-41.4,;48.08,-42.63,;48.08,-44.22,;49.49,-44.78,;50.5,-43.51,;52.04,-43.51,;50.51,-41.98,;49.11,-43.85,;41.82,-44.51,;42.6,-43.18,;41.83,-41.83,;40.28,-41.83,;39.5,-43.16,;40.27,-44.5,)| Show InChI InChI=1S/C30H38N6O3/c1-20(37)33-8-10-34(11-9-33)24-6-7-27(31-19-24)35-12-13-36(26-5-3-2-4-25(26)35)29(38)32-28-22-14-21-15-23(28)18-30(39,16-21)17-22/h2-7,19,21-23,28,39H,8-18H2,1H3,(H,32,38)/t21?,22?,23?,28-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438092
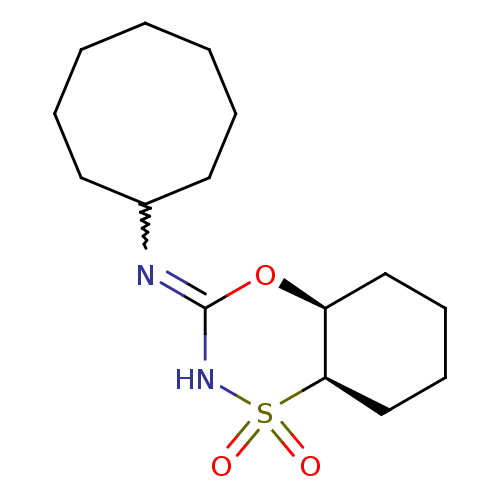
(CHEMBL2409743)Show SMILES O=S1(=O)NC(O[C@H]2CCCC[C@@H]12)=NC1CCCCCCC1 |r,w:12.14| Show InChI InChI=1S/C15H26N2O3S/c18-21(19)14-11-7-6-10-13(14)20-15(17-21)16-12-8-4-2-1-3-5-9-12/h12-14H,1-11H2,(H,16,17)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433404

(CHEMBL2380641)Show SMILES CS(=O)(=O)N1CCN(CC1)c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(2.53,-27.78,;3.31,-29.1,;3.3,-30.64,;1.97,-29.87,;4.85,-29.11,;5.62,-30.43,;7.15,-30.43,;7.92,-29.1,;7.15,-27.77,;5.61,-27.78,;9.45,-29.1,;10.22,-30.44,;11.76,-30.44,;12.53,-29.1,;11.75,-27.76,;10.22,-27.77,;14.07,-29.1,;14.84,-30.44,;16.37,-30.44,;17.15,-29.11,;18.69,-29.12,;19.46,-30.45,;19.61,-27.88,;21.15,-27.91,;22.34,-26.63,;22.33,-25.15,;23.68,-24.67,;22.64,-25.9,;22.65,-27.49,;24.05,-28.05,;25.07,-26.78,;26.61,-26.78,;25.08,-25.25,;23.67,-27.12,;16.38,-27.78,;17.16,-26.45,;16.4,-25.1,;14.84,-25.1,;14.07,-26.43,;14.84,-27.77,)| Show InChI InChI=1S/C28H37N7O4S/c1-40(38,39)33-8-6-32(7-9-33)22-17-29-26(30-18-22)34-10-11-35(24-5-3-2-4-23(24)34)27(36)31-25-20-12-19-13-21(25)16-28(37,14-19)15-20/h2-5,17-21,25,37H,6-16H2,1H3,(H,31,36)/t19?,20?,21?,25-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438071
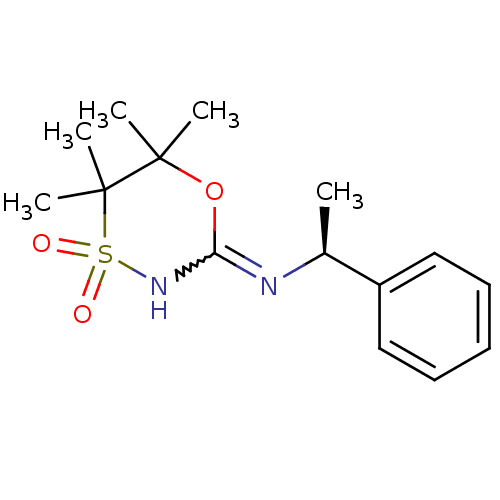
(CHEMBL2409723)Show SMILES C[C@H](N=C1NS(=O)(=O)C(C)(C)C(C)(C)O1)c1ccccc1 |r,w:3.3| Show InChI InChI=1S/C15H22N2O3S/c1-11(12-9-7-6-8-10-12)16-13-17-21(18,19)15(4,5)14(2,3)20-13/h6-11H,1-5H3,(H,16,17)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433400

(CHEMBL2380637)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:21.22,wD:28.31,TLB:20:21:31.27.28:25.24.23,20:21:23:31.28.30,29:28:21.26.25:23,THB:27:26:23:31.28.30,27:28:21.26.25:23,29:28:21:25.24.23,30:28:21:25.24.23,30:24:21:31.27.28,(.2,-12.39,;.98,-13.73,;2.52,-13.72,;3.29,-15.05,;4.83,-15.05,;5.59,-13.72,;4.83,-12.39,;3.28,-12.39,;7.13,-13.71,;7.9,-15.05,;9.44,-15.05,;10.21,-13.72,;9.43,-12.38,;7.9,-12.38,;11.76,-13.72,;12.53,-15.06,;14.06,-15.06,;14.84,-13.73,;16.38,-13.73,;17.15,-15.07,;17.3,-12.49,;18.84,-12.52,;20.04,-11.24,;20.03,-9.76,;21.38,-9.28,;20.34,-10.51,;20.35,-12.1,;21.75,-12.67,;22.77,-11.39,;24.31,-11.33,;22.78,-9.86,;21.37,-11.73,;14.07,-12.39,;14.85,-11.06,;14.09,-9.71,;12.53,-9.71,;11.76,-11.04,;12.52,-12.38,)| Show InChI InChI=1S/C30H33N5O3/c1-38-24-8-6-20(7-9-24)23-17-31-28(32-18-23)34-10-11-35(26-5-3-2-4-25(26)34)29(36)33-27-21-12-19-13-22(27)16-30(37,14-19)15-21/h2-9,17-19,21-22,27,37H,10-16H2,1H3,(H,33,36)/t19?,21?,22?,27-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50433405

(CHEMBL2380642)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(25.74,-29.77,;26.51,-31.1,;26.5,-32.64,;25.17,-31.86,;28.05,-31.1,;28.82,-32.42,;30.36,-32.43,;31.12,-31.1,;30.35,-29.77,;28.81,-29.77,;32.66,-31.09,;33.42,-32.43,;34.96,-32.43,;35.73,-31.1,;34.96,-29.76,;33.42,-29.76,;37.28,-31.1,;38.05,-32.43,;39.58,-32.44,;40.35,-31.11,;41.89,-31.11,;42.66,-32.45,;42.82,-29.88,;44.35,-29.9,;45.55,-28.63,;45.54,-27.14,;46.89,-26.66,;45.85,-27.9,;45.85,-29.48,;47.26,-30.05,;48.27,-28.77,;49.81,-28.77,;48.28,-27.24,;46.88,-29.12,;39.59,-29.77,;40.37,-28.44,;39.6,-27.1,;38.05,-27.09,;37.28,-28.43,;38.04,-29.76,)| Show InChI InChI=1S/C29H38N6O4S/c1-40(38,39)33-10-8-32(9-11-33)23-6-7-26(30-19-23)34-12-13-35(25-5-3-2-4-24(25)34)28(36)31-27-21-14-20-15-22(27)18-29(37,16-20)17-21/h2-7,19-22,27,37H,8-18H2,1H3,(H,31,36)/t20?,21?,22?,27-,29- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438086
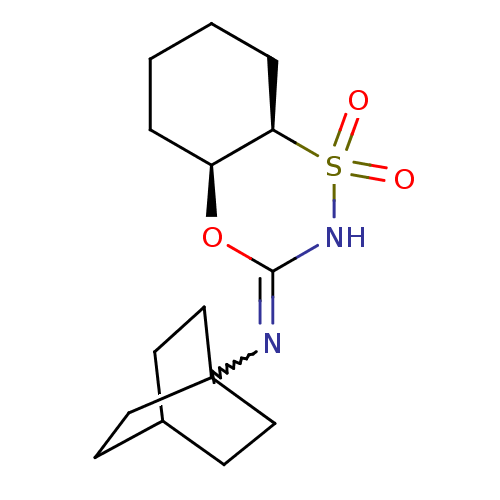
(CHEMBL2409752)Show SMILES O=S1(=O)NC(O[C@H]2CCCC[C@@H]12)=NC12CCC(CC1)CC2 |r,w:12.14| Show InChI InChI=1S/C15H24N2O3S/c18-21(19)13-4-2-1-3-12(13)20-14(17-21)16-15-8-5-11(6-9-15)7-10-15/h11-13H,1-10H2,(H,16,17)/t11?,12-,13+,15?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50438077

(CHEMBL2409612)Show SMILES OCC[C@H](N=C1NS(=O)(=O)[C@@H]2CCCC[C@@H]2O1)c1ccccc1 |r,w:5.5| Show InChI InChI=1S/C16H22N2O4S/c19-11-10-13(12-6-2-1-3-7-12)17-16-18-23(20,21)15-9-5-4-8-14(15)22-16/h1-3,6-7,13-15,19H,4-5,8-11H2,(H,17,18)/t13-,14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438105
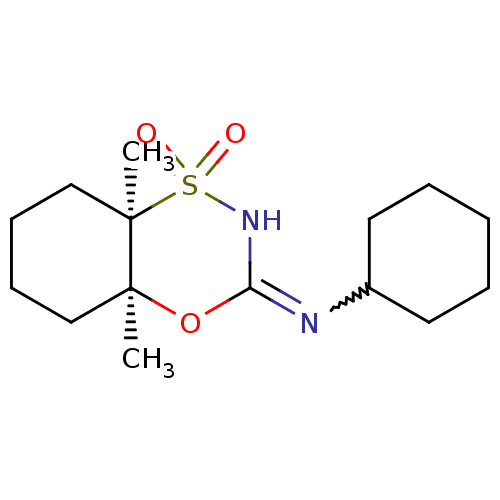
(CHEMBL2409740)Show SMILES C[C@]12CCCC[C@@]1(C)S(=O)(=O)NC(O2)=NC1CCCCC1 |r,w:14.16| Show InChI InChI=1S/C15H26N2O3S/c1-14-10-6-7-11-15(14,2)21(18,19)17-13(20-14)16-12-8-4-3-5-9-12/h12H,3-11H2,1-2H3,(H,16,17)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433402

(CHEMBL2380639)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:21.22,wD:28.35,TLB:31:28:21.26.25:23,20:21:28.30.27:25.24.23,20:21:23:28.30.29,THB:27:28:21.26.25:23,27:26:23:28.30.29,29:28:21:25.24.23,29:24:21:28.30.27,(1.22,-19.65,;2,-20.98,;3.54,-20.98,;4.3,-22.3,;5.84,-22.3,;6.61,-20.98,;5.84,-19.65,;4.3,-19.65,;8.14,-20.97,;8.91,-22.31,;10.45,-22.31,;11.22,-20.98,;10.44,-19.64,;8.91,-19.64,;12.76,-20.97,;13.53,-22.31,;15.07,-22.32,;15.84,-20.98,;17.38,-20.99,;18.15,-22.32,;18.3,-19.76,;19.84,-19.78,;21.04,-18.5,;21.03,-17.02,;22.38,-16.54,;21.34,-17.78,;21.34,-19.36,;22.75,-19.93,;23.76,-18.65,;23.77,-17.12,;22.37,-19,;25.3,-18.65,;26.07,-19.98,;26.07,-17.31,;15.08,-19.65,;15.86,-18.32,;15.09,-16.97,;13.53,-16.97,;12.76,-18.31,;13.53,-19.64,)| Show InChI InChI=1S/C31H34N6O3/c1-40-24-8-6-20(7-9-24)23-17-33-29(34-18-23)36-10-11-37(26-5-3-2-4-25(26)36)30(39)35-27-21-12-19-13-22(27)16-31(14-19,15-21)28(32)38/h2-9,17-19,21-22,27H,10-16H2,1H3,(H2,32,38)(H,35,39)/t19?,21?,22?,27-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438075
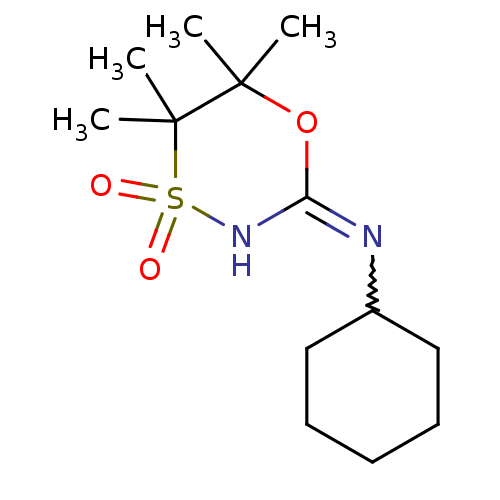
(CHEMBL2409617)Show SMILES CC1(C)OC(NS(=O)(=O)C1(C)C)=NC1CCCCC1 |w:12.13| Show InChI InChI=1S/C13H24N2O3S/c1-12(2)13(3,4)19(16,17)15-11(18-12)14-10-8-6-5-7-9-10/h10H,5-9H2,1-4H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340440

((3-(1H-pyrazol-4-yl)pyrrolidin-1-yl)(2',3'-dihydro...)Show SMILES O=C(N1CCC(C1)c1cn[nH]c1)N1CCC2(CC2)c2ccccc12 Show InChI InChI=1S/C19H22N4O/c24-18(22-9-5-14(13-22)15-11-20-21-12-15)23-10-8-19(6-7-19)16-3-1-2-4-17(16)23/h1-4,11-12,14H,5-10,13H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 assessed as conversion of radiolabelled-cortisone to cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50433408

(CHEMBL2380645)Show SMILES CC(C)(C)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(1.87,-44.23,;2.65,-45.56,;1.88,-46.9,;1.1,-45.55,;4.19,-45.56,;4.95,-46.88,;6.49,-46.88,;7.26,-45.55,;6.49,-44.22,;4.95,-44.23,;8.79,-45.55,;9.56,-44.22,;11.09,-44.21,;11.87,-45.55,;11.1,-46.89,;9.56,-46.89,;13.41,-45.55,;14.18,-46.89,;15.71,-46.89,;16.49,-45.56,;18.03,-45.57,;18.8,-46.9,;18.95,-44.33,;20.49,-44.36,;21.68,-43.08,;21.67,-41.6,;23.02,-41.12,;21.98,-42.35,;21.99,-43.94,;23.39,-44.5,;24.41,-43.23,;25.95,-43.23,;24.42,-41.7,;23.01,-43.57,;15.72,-44.23,;16.5,-42.9,;15.74,-41.55,;14.18,-41.55,;13.41,-42.88,;14.17,-44.22,)| Show InChI InChI=1S/C32H44N6O2/c1-31(2,3)36-12-10-35(11-13-36)25-8-9-28(33-21-25)37-14-15-38(27-7-5-4-6-26(27)37)30(39)34-29-23-16-22-17-24(29)20-32(40,18-22)19-23/h4-9,21-24,29,40H,10-20H2,1-3H3,(H,34,39)/t22?,23?,24?,29-,32- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data