

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of [1-beta-2beta-3H]- -testosterone binding to human steroid 5-alpha-reductase type 2 of BPH tissue at 10 uM | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50063477 ((3S,10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coli | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of [1-beta-3H]-androstenedione binding to human steroid 5-alpha-reductase type I expressed in DU-145 cells at 10 uM | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128564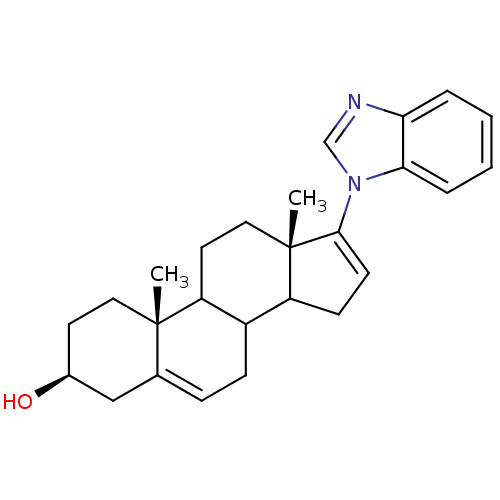 ((3S,10R,13S)-17-Benzoimidazol-1-yl-10,13-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coli | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50128544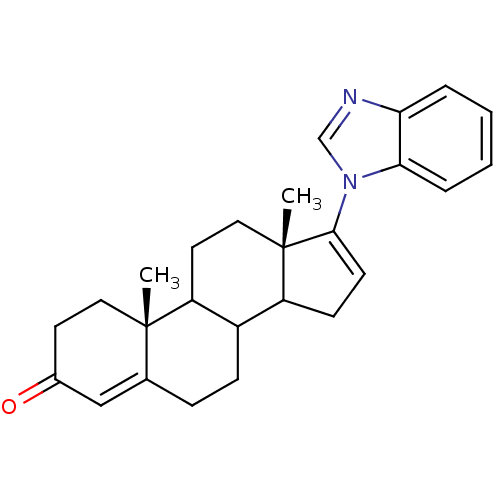 ((10R,13S)-17-Benzoimidazol-1-yl-10,13-dimethyl-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of [1-beta-2beta-3H]- -testosterone binding to human steroid 5-alpha-reductase type 2 of BPH tissue at 10 uM | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128543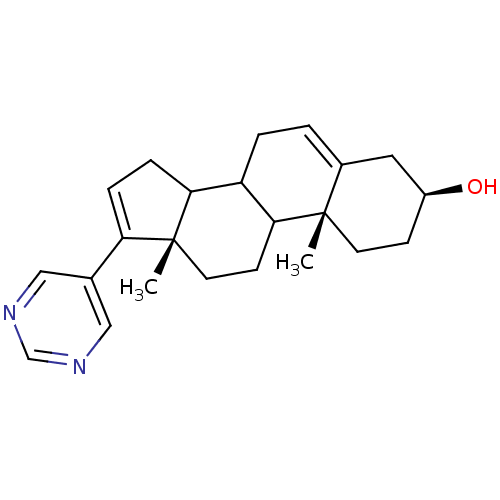 ((3S,10R,13S)-10,13-Dimethyl-17-pyrimidin-5-yl-2,3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coli | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50128544 ((10R,13S)-17-Benzoimidazol-1-yl-10,13-dimethyl-1,2...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of [1-beta-3H]-androstenedione binding to human steroid 5-alpha-reductase type I expressed in DU-145 cells at 10 uM | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coli | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128544 ((10R,13S)-17-Benzoimidazol-1-yl-10,13-dimethyl-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 915 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coli | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM31768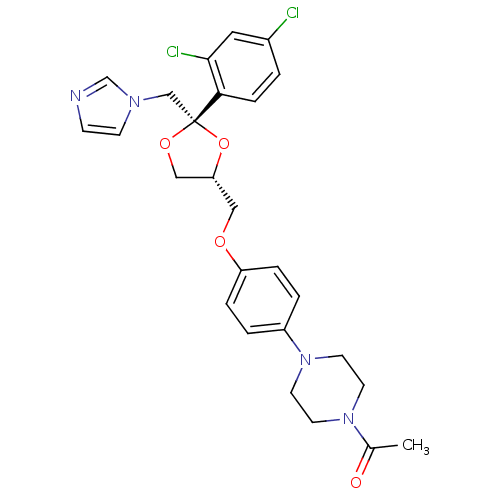 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coli | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128552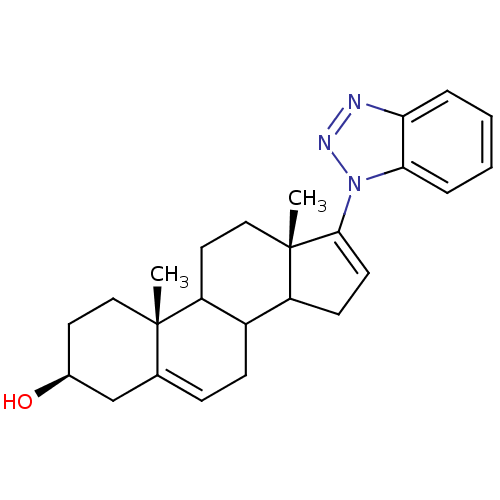 ((3S,10R,13S)-17-Benzotriazol-1-yl-10,13-dimethyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coli | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128542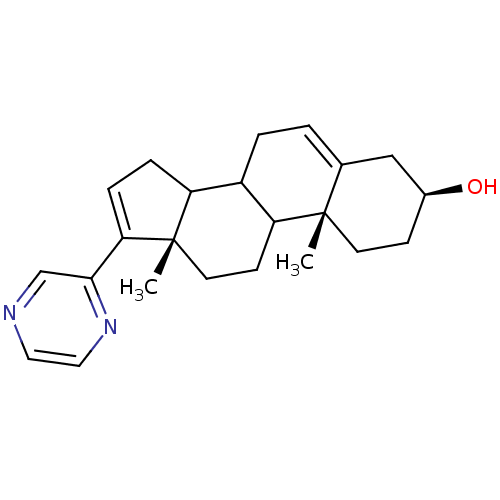 ((3S,10R,13S)-10,13-Dimethyl-17-pyrazin-2-yl-2,3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coli | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50165357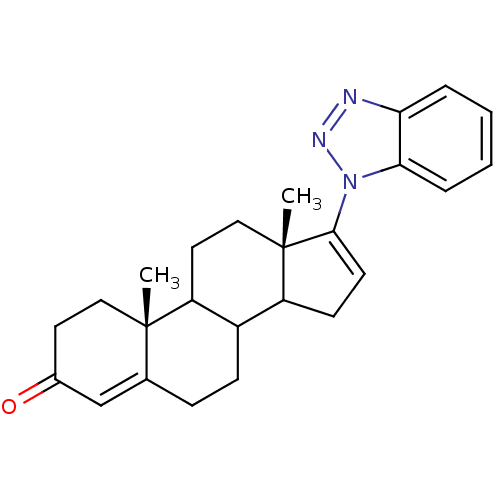 ((10R,13S)-17-Benzotriazol-1-yl-10,13-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coli | J Med Chem 48: 2972-84 (2005) Article DOI: 10.1021/jm040202w BindingDB Entry DOI: 10.7270/Q2TH8NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||