 Found 581 hits with Last Name = 'farrelly' and Initial = 'd'
Found 581 hits with Last Name = 'farrelly' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50248410
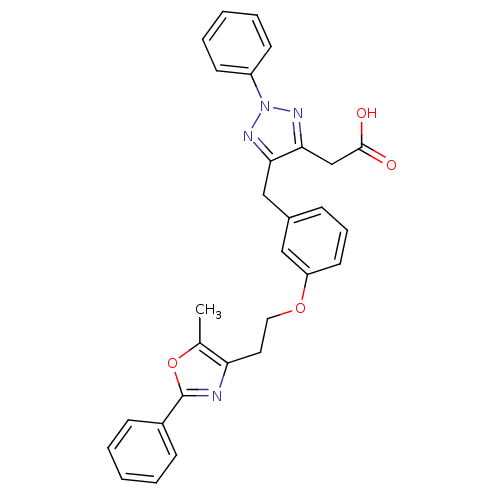
((5-{3-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy...)Show SMILES Cc1oc(nc1CCOc1cccc(Cc2nn(nc2CC(O)=O)-c2ccccc2)c1)-c1ccccc1 Show InChI InChI=1S/C29H26N4O4/c1-20-25(30-29(37-20)22-10-4-2-5-11-22)15-16-36-24-14-8-9-21(17-24)18-26-27(19-28(34)35)32-33(31-26)23-12-6-3-7-13-23/h2-14,17H,15-16,18-19H2,1H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50248463
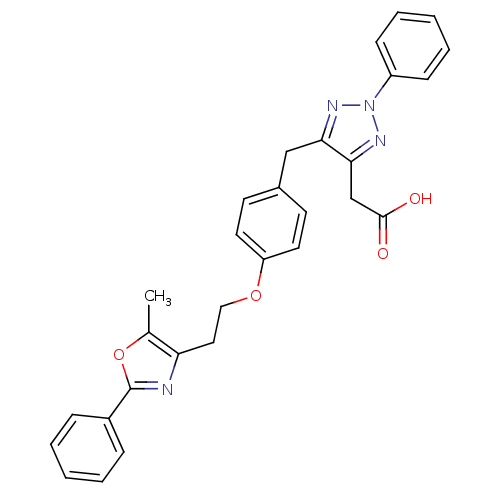
(2-(5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)be...)Show SMILES Cc1oc(nc1CCOc1ccc(Cc2nn(nc2CC(O)=O)-c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C29H26N4O4/c1-20-25(30-29(37-20)22-8-4-2-5-9-22)16-17-36-24-14-12-21(13-15-24)18-26-27(19-28(34)35)32-33(31-26)23-10-6-3-7-11-23/h2-15H,16-19H2,1H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50377310

(CHEMBL255309)Show SMILES Cc1oc(nc1CCOc1cccc(c1)C1=C(CC(O)=O)CCN(C1)C(=O)Oc1ccccc1)-c1ccccc1 |c:17| Show InChI InChI=1S/C32H30N2O6/c1-22-29(33-31(39-22)23-9-4-2-5-10-23)16-18-38-27-14-8-11-24(19-27)28-21-34(17-15-25(28)20-30(35)36)32(37)40-26-12-6-3-7-13-26/h2-14,19H,15-18,20-21H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Binding affinity at human PPARalpha by fluorescence polarization |
Bioorg Med Chem Lett 18: 3545-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.014
BindingDB Entry DOI: 10.7270/Q2RX9D04 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50063315
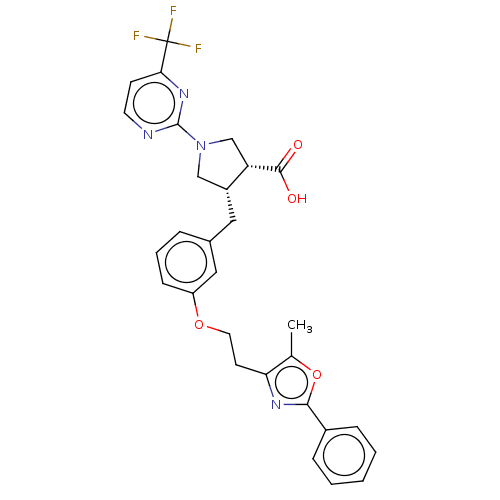
(CHEMBL3398446)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2nccc(n2)C(F)(F)F)c1)-c1ccccc1 |r| Show InChI InChI=1S/C29H27F3N4O4/c1-18-24(34-26(40-18)20-7-3-2-4-8-20)11-13-39-22-9-5-6-19(15-22)14-21-16-36(17-23(21)27(37)38)28-33-12-10-25(35-28)29(30,31)32/h2-10,12,15,21,23H,11,13-14,16-17H2,1H3,(H,37,38)/t21-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50377302
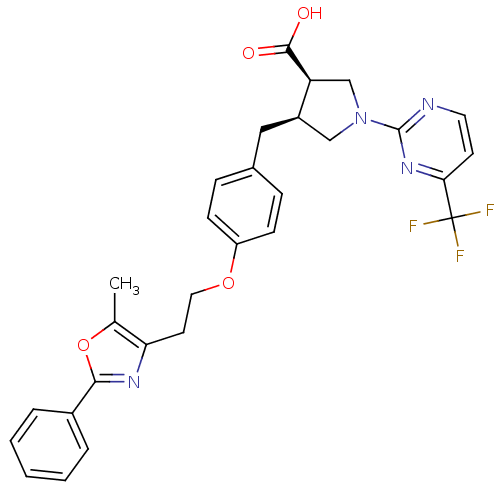
(CHEMBL255930)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2nccc(n2)C(F)(F)F)cc1)-c1ccccc1 Show InChI InChI=1S/C29H27F3N4O4/c1-18-24(34-26(40-18)20-5-3-2-4-6-20)12-14-39-22-9-7-19(8-10-22)15-21-16-36(17-23(21)27(37)38)28-33-13-11-25(35-28)29(30,31)32/h2-11,13,21,23H,12,14-17H2,1H3,(H,37,38)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50377302

(CHEMBL255930)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2nccc(n2)C(F)(F)F)cc1)-c1ccccc1 Show InChI InChI=1S/C29H27F3N4O4/c1-18-24(34-26(40-18)20-5-3-2-4-6-20)12-14-39-22-9-7-19(8-10-22)15-21-16-36(17-23(21)27(37)38)28-33-13-11-25(35-28)29(30,31)32/h2-11,13,21,23H,12,14-17H2,1H3,(H,37,38)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50205086

(2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...)Show SMILES Cc1oc(nc1CCOc1cccc(CN(CC(O)=O)Cc2cccc3ccccc23)c1)-c1ccccc1 Show InChI InChI=1S/C32H30N2O4/c1-23-30(33-32(38-23)26-11-3-2-4-12-26)17-18-37-28-15-7-9-24(19-28)20-34(22-31(35)36)21-27-14-8-13-25-10-5-6-16-29(25)27/h2-16,19H,17-18,20-22H2,1H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50248409
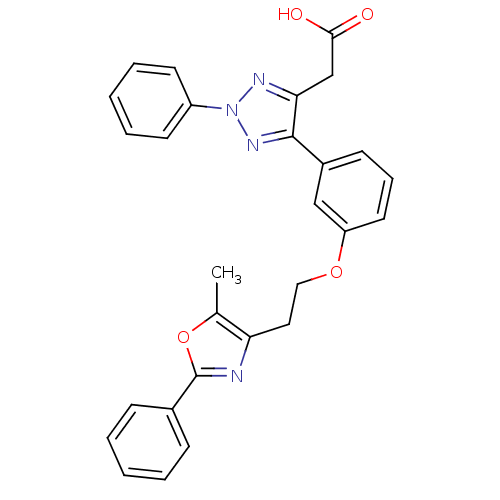
(2-(5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)ph...)Show SMILES Cc1oc(nc1CCOc1cccc(c1)-c1nn(nc1CC(O)=O)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C28H24N4O4/c1-19-24(29-28(36-19)20-9-4-2-5-10-20)15-16-35-23-14-8-11-21(17-23)27-25(18-26(33)34)30-32(31-27)22-12-6-3-7-13-22/h2-14,17H,15-16,18H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50377310

(CHEMBL255309)Show SMILES Cc1oc(nc1CCOc1cccc(c1)C1=C(CC(O)=O)CCN(C1)C(=O)Oc1ccccc1)-c1ccccc1 |c:17| Show InChI InChI=1S/C32H30N2O6/c1-22-29(33-31(39-22)23-9-4-2-5-10-23)16-18-38-27-14-8-11-24(19-27)28-21-34(17-15-25(28)20-30(35)36)32(37)40-26-12-6-3-7-13-26/h2-14,19H,15-18,20-21H2,1H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Binding affinity at human PPARalpha by fluorescence polarization |
Bioorg Med Chem Lett 18: 3545-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.014
BindingDB Entry DOI: 10.7270/Q2RX9D04 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50063311

(CHEMBL3398444)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2ccccc2)c1)-c1ccccc1 |r| Show InChI InChI=1S/C30H30N2O4/c1-21-28(31-29(36-21)23-10-4-2-5-11-23)15-16-35-26-14-8-9-22(18-26)17-24-19-32(20-27(24)30(33)34)25-12-6-3-7-13-25/h2-14,18,24,27H,15-17,19-20H2,1H3,(H,33,34)/t24-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50063311

(CHEMBL3398444)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2ccccc2)c1)-c1ccccc1 |r| Show InChI InChI=1S/C30H30N2O4/c1-21-28(31-29(36-21)23-10-4-2-5-11-23)15-16-35-26-14-8-9-22(18-26)17-24-19-32(20-27(24)30(33)34)25-12-6-3-7-13-25/h2-14,18,24,27H,15-17,19-20H2,1H3,(H,33,34)/t24-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372118

(CHEMBL255389)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-10-6-5-7-11-23)17-18-39-26-12-8-9-22(19-26)20-27-29(32(37)38)35(31(27)36)25-15-13-24(14-16-25)33(2,3)4/h5-16,19,27,29H,17-18,20H2,1-4H3,(H,37,38)/t27-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063315

(CHEMBL3398446)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2nccc(n2)C(F)(F)F)c1)-c1ccccc1 |r| Show InChI InChI=1S/C29H27F3N4O4/c1-18-24(34-26(40-18)20-7-3-2-4-8-20)11-13-39-22-9-5-6-19(15-22)14-21-16-36(17-23(21)27(37)38)28-33-12-10-25(35-28)29(30,31)32/h2-10,12,15,21,23H,11,13-14,16-17H2,1H3,(H,37,38)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372105

(CHEMBL411118)Show SMILES COc1ccc(cc1)N1[C@@H]([C@H](Cc2ccc(OCCc3nc(oc3C)-c3ccccc3)cc2)C1=O)C(O)=O Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-6-4-3-5-7-21)16-17-37-24-12-8-20(9-13-24)18-25-27(30(34)35)32(29(25)33)22-10-14-23(36-2)15-11-22/h3-15,25,27H,16-18H2,1-2H3,(H,34,35)/t25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50377311

(CHEMBL403432)Show SMILES CC(C)COC(=O)N1CCC(CC(O)=O)=C(C1)c1cccc(OCCc2nc(oc2C)-c2ccccc2)c1 |c:14| Show InChI InChI=1S/C30H34N2O6/c1-20(2)19-37-30(35)32-14-12-24(17-28(33)34)26(18-32)23-10-7-11-25(16-23)36-15-13-27-21(3)38-29(31-27)22-8-5-4-6-9-22/h4-11,16,20H,12-15,17-19H2,1-3H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Binding affinity at human PPARalpha by fluorescence polarization |
Bioorg Med Chem Lett 18: 3545-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.014
BindingDB Entry DOI: 10.7270/Q2RX9D04 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063313

(CHEMBL3398454)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2nc(co2)C(F)(F)F)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C28H26F3N3O5/c1-17-23(32-25(39-17)19-5-3-2-4-6-19)11-12-37-21-9-7-18(8-10-21)13-20-14-34(15-22(20)26(35)36)27-33-24(16-38-27)28(29,30)31/h2-10,16,20,22H,11-15H2,1H3,(H,35,36)/t20-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50205080
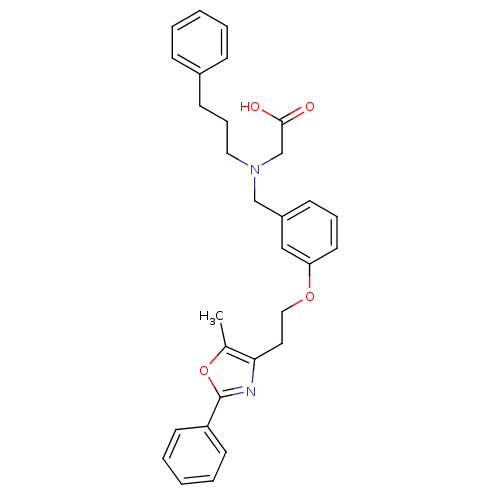
(2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...)Show SMILES Cc1oc(nc1CCOc1cccc(CN(CCCc2ccccc2)CC(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C30H32N2O4/c1-23-28(31-30(36-23)26-14-6-3-7-15-26)17-19-35-27-16-8-12-25(20-27)21-32(22-29(33)34)18-9-13-24-10-4-2-5-11-24/h2-8,10-12,14-16,20H,9,13,17-19,21-22H2,1H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50248463

(2-(5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)be...)Show SMILES Cc1oc(nc1CCOc1ccc(Cc2nn(nc2CC(O)=O)-c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C29H26N4O4/c1-20-25(30-29(37-20)22-8-4-2-5-9-22)16-17-36-24-14-12-21(13-15-24)18-26-27(19-28(34)35)32-33(31-26)23-10-6-3-7-11-23/h2-15H,16-19H2,1H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50205086

(2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...)Show SMILES Cc1oc(nc1CCOc1cccc(CN(CC(O)=O)Cc2cccc3ccccc23)c1)-c1ccccc1 Show InChI InChI=1S/C32H30N2O4/c1-23-30(33-32(38-23)26-11-3-2-4-12-26)17-18-37-28-15-7-9-24(19-28)20-34(22-31(35)36)21-27-14-8-13-25-10-5-6-16-29(25)27/h2-16,19H,17-18,20-22H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50248288

(5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...)Show SMILES Cc1oc(nc1CCOc1cccc(Cc2nn(nc2C(O)=O)-c2ccccc2)c1)-c1ccccc1 Show InChI InChI=1S/C28H24N4O4/c1-19-24(29-27(36-19)21-10-4-2-5-11-21)15-16-35-23-14-8-9-20(17-23)18-25-26(28(33)34)31-32(30-25)22-12-6-3-7-13-22/h2-14,17H,15-16,18H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50248288

(5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...)Show SMILES Cc1oc(nc1CCOc1cccc(Cc2nn(nc2C(O)=O)-c2ccccc2)c1)-c1ccccc1 Show InChI InChI=1S/C28H24N4O4/c1-19-24(29-27(36-19)21-10-4-2-5-11-21)15-16-35-23-14-8-9-20(17-23)18-25-26(28(33)34)31-32(30-25)22-12-6-3-7-13-22/h2-14,17H,15-16,18H2,1H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50248226
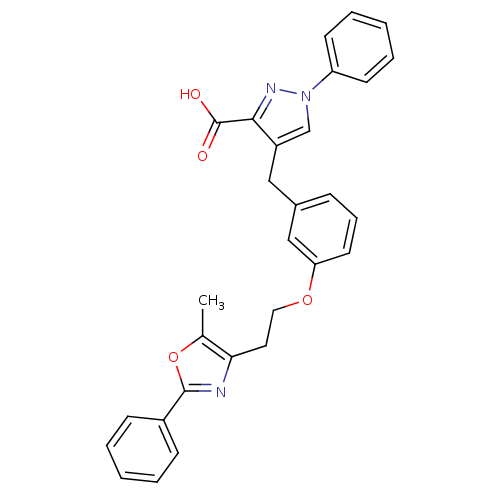
(4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...)Show SMILES Cc1oc(nc1CCOc1cccc(Cc2cn(nc2C(O)=O)-c2ccccc2)c1)-c1ccccc1 Show InChI InChI=1S/C29H25N3O4/c1-20-26(30-28(36-20)22-10-4-2-5-11-22)15-16-35-25-14-8-9-21(18-25)17-23-19-32(31-27(23)29(33)34)24-12-6-3-7-13-24/h2-14,18-19H,15-17H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50248224
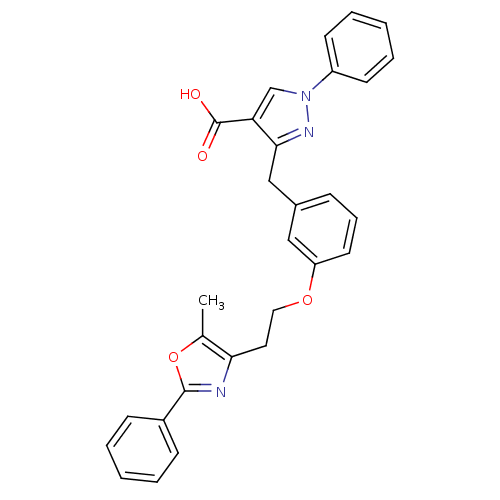
(3-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...)Show SMILES Cc1oc(nc1CCOc1cccc(Cc2nn(cc2C(O)=O)-c2ccccc2)c1)-c1ccccc1 Show InChI InChI=1S/C29H25N3O4/c1-20-26(30-28(36-20)22-10-4-2-5-11-22)15-16-35-24-14-8-9-21(17-24)18-27-25(29(33)34)19-32(31-27)23-12-6-3-7-13-23/h2-14,17,19H,15-16,18H2,1H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50205081

(2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...)Show SMILES Cc1oc(nc1CCOc1cccc(CN(CCc2ccccc2)CC(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C29H30N2O4/c1-22-27(30-29(35-22)25-12-6-3-7-13-25)16-18-34-26-14-8-11-24(19-26)20-31(21-28(32)33)17-15-23-9-4-2-5-10-23/h2-14,19H,15-18,20-21H2,1H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372118

(CHEMBL255389)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-10-6-5-7-11-23)17-18-39-26-12-8-9-22(19-26)20-27-29(32(37)38)35(31(27)36)25-15-13-24(14-16-25)33(2,3)4/h5-16,19,27,29H,17-18,20H2,1-4H3,(H,37,38)/t27-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063089

(CHEMBL3398443)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2CN(C[C@@H]2C(O)=O)C(=O)Oc2ccccc2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H30N2O6/c1-21-28(32-29(38-21)23-8-4-2-5-9-23)16-17-37-25-14-12-22(13-15-25)18-24-19-33(20-27(24)30(34)35)31(36)39-26-10-6-3-7-11-26/h2-15,24,27H,16-20H2,1H3,(H,34,35)/t24-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063089

(CHEMBL3398443)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2CN(C[C@@H]2C(O)=O)C(=O)Oc2ccccc2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H30N2O6/c1-21-28(32-29(38-21)23-8-4-2-5-9-23)16-17-37-25-14-12-22(13-15-25)18-24-19-33(20-27(24)30(34)35)31(36)39-26-10-6-3-7-11-26/h2-15,24,27H,16-20H2,1H3,(H,34,35)/t24-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063311

(CHEMBL3398444)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2ccccc2)c1)-c1ccccc1 |r| Show InChI InChI=1S/C30H30N2O4/c1-21-28(31-29(36-21)23-10-4-2-5-11-23)15-16-35-26-14-8-9-22(18-26)17-24-19-32(20-27(24)30(33)34)25-12-6-3-7-13-25/h2-14,18,24,27H,15-17,19-20H2,1H3,(H,33,34)/t24-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063311

(CHEMBL3398444)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2ccccc2)c1)-c1ccccc1 |r| Show InChI InChI=1S/C30H30N2O4/c1-21-28(31-29(36-21)23-10-4-2-5-11-23)15-16-35-26-14-8-9-22(18-26)17-24-19-32(20-27(24)30(33)34)25-12-6-3-7-13-25/h2-14,18,24,27H,15-17,19-20H2,1H3,(H,33,34)/t24-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50205089

(2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...)Show SMILES Cc1oc(nc1CCOc1cccc(CN(CC(O)=O)Cc2cccc(Oc3ccccc3)c2)c1)-c1ccccc1 Show InChI InChI=1S/C34H32N2O5/c1-25-32(35-34(40-25)28-12-4-2-5-13-28)18-19-39-30-16-8-10-26(20-30)22-36(24-33(37)38)23-27-11-9-17-31(21-27)41-29-14-6-3-7-15-29/h2-17,20-21H,18-19,22-24H2,1H3,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50248410

((5-{3-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy...)Show SMILES Cc1oc(nc1CCOc1cccc(Cc2nn(nc2CC(O)=O)-c2ccccc2)c1)-c1ccccc1 Show InChI InChI=1S/C29H26N4O4/c1-20-25(30-29(37-20)22-10-4-2-5-11-22)15-16-36-24-14-8-9-21(17-24)18-26-27(19-28(34)35)32-33(31-26)23-12-6-3-7-13-23/h2-14,17H,15-16,18-19H2,1H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
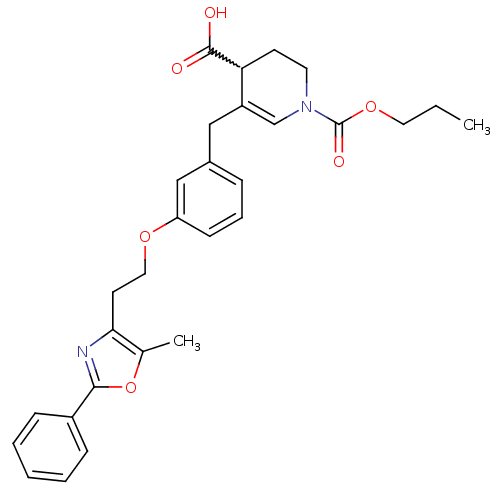
(Homo sapiens (Human)) | BDBM50377303
(CHEMBL256142)Show SMILES CCCOC(=O)N1CCC(C(O)=O)C(Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)=C1 |w:9.9,c:38| Show InChI InChI=1S/C29H32N2O6/c1-3-15-36-29(34)31-14-12-25(28(32)33)23(19-31)17-21-8-7-11-24(18-21)35-16-13-26-20(2)37-27(30-26)22-9-5-4-6-10-22/h4-11,18-19,25H,3,12-17H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Binding affinity at human PPARalpha by fluorescence polarization |
Bioorg Med Chem Lett 18: 3545-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.014
BindingDB Entry DOI: 10.7270/Q2RX9D04 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50205072
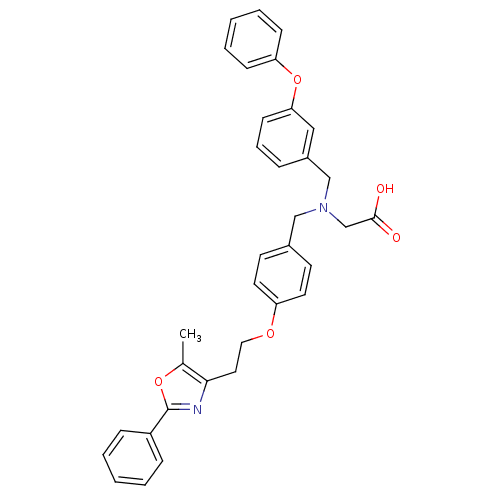
(2-((3-phenoxybenzyl)(4-(2-(5-methyl-2-phenyloxazol...)Show SMILES Cc1oc(nc1CCOc1ccc(CN(CC(O)=O)Cc2cccc(Oc3ccccc3)c2)cc1)-c1ccccc1 Show InChI InChI=1S/C34H32N2O5/c1-25-32(35-34(40-25)28-10-4-2-5-11-28)19-20-39-29-17-15-26(16-18-29)22-36(24-33(37)38)23-27-9-8-14-31(21-27)41-30-12-6-3-7-13-30/h2-18,21H,19-20,22-24H2,1H3,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50205075
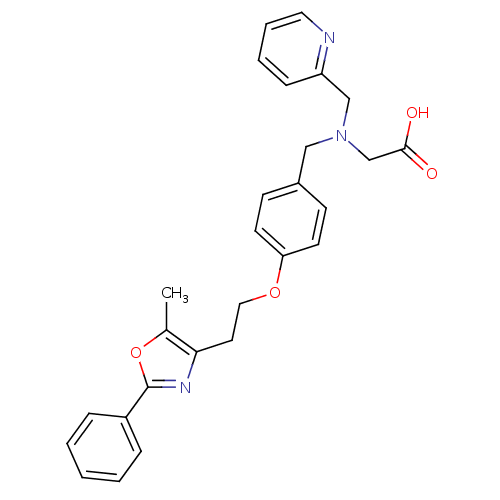
(2-((4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...)Show SMILES Cc1oc(nc1CCOc1ccc(CN(CC(O)=O)Cc2ccccn2)cc1)-c1ccccc1 Show InChI InChI=1S/C27H27N3O4/c1-20-25(29-27(34-20)22-7-3-2-4-8-22)14-16-33-24-12-10-21(11-13-24)17-30(19-26(31)32)18-23-9-5-6-15-28-23/h2-13,15H,14,16-19H2,1H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50248179
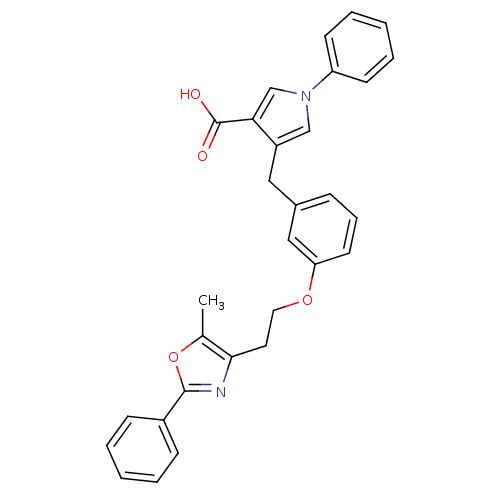
(4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...)Show SMILES Cc1oc(nc1CCOc1cccc(Cc2cn(cc2C(O)=O)-c2ccccc2)c1)-c1ccccc1 Show InChI InChI=1S/C30H26N2O4/c1-21-28(31-29(36-21)23-10-4-2-5-11-23)15-16-35-26-14-8-9-22(18-26)17-24-19-32(20-27(24)30(33)34)25-12-6-3-7-13-25/h2-14,18-20H,15-17H2,1H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50205088

(2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...)Show SMILES Cc1oc(nc1CCOc1cccc(CN(CC(O)=O)Cc2ccccc2)c1)-c1ccccc1 Show InChI InChI=1S/C28H28N2O4/c1-21-26(29-28(34-21)24-12-6-3-7-13-24)15-16-33-25-14-8-11-23(17-25)19-30(20-27(31)32)18-22-9-4-2-5-10-22/h2-14,17H,15-16,18-20H2,1H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063099
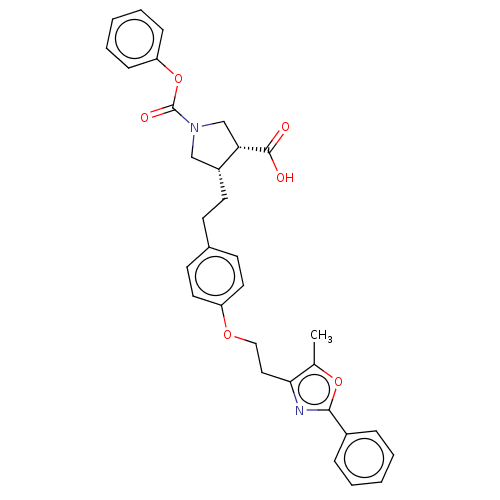
(CHEMBL3398459)Show SMILES Cc1oc(nc1CCOc1ccc(CC[C@@H]2CN(C[C@@H]2C(O)=O)C(=O)Oc2ccccc2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C32H32N2O6/c1-22-29(33-30(39-22)24-8-4-2-5-9-24)18-19-38-26-16-13-23(14-17-26)12-15-25-20-34(21-28(25)31(35)36)32(37)40-27-10-6-3-7-11-27/h2-11,13-14,16-17,25,28H,12,15,18-21H2,1H3,(H,35,36)/t25-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372107

(CHEMBL271240)Show SMILES COc1ccc(cc1)N1[C@@H]([C@H](Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)C1=O)C(O)=O |r| Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-8-4-3-5-9-21)15-16-37-24-10-6-7-20(17-24)18-25-27(30(34)35)32(29(25)33)22-11-13-23(36-2)14-12-22/h3-14,17,25,27H,15-16,18H2,1-2H3,(H,34,35)/t25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50205079

(2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...)Show SMILES Cc1oc(nc1CCOc1cccc(CN(CC(O)=O)Cc2ccc(Oc3ccccc3)cc2)c1)-c1ccccc1 Show InChI InChI=1S/C34H32N2O5/c1-25-32(35-34(40-25)28-10-4-2-5-11-28)19-20-39-31-14-8-9-27(21-31)23-36(24-33(37)38)22-26-15-17-30(18-16-26)41-29-12-6-3-7-13-29/h2-18,21H,19-20,22-24H2,1H3,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372116
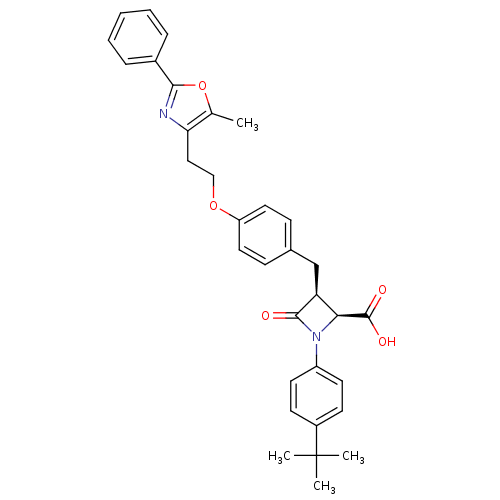
(CHEMBL256468)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-8-6-5-7-9-23)18-19-39-26-16-10-22(11-17-26)20-27-29(32(37)38)35(31(27)36)25-14-12-24(13-15-25)33(2,3)4/h5-17,27,29H,18-20H2,1-4H3,(H,37,38)/t27-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372114
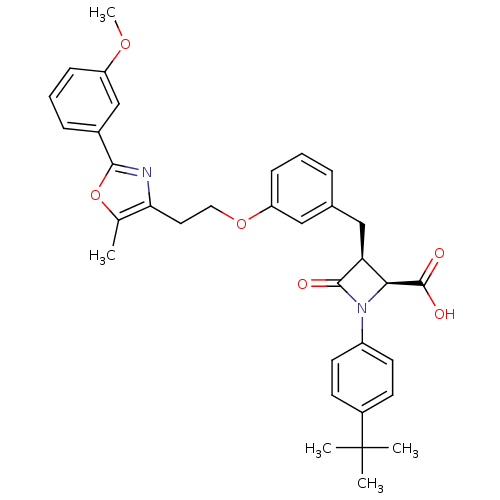
(CHEMBL404646)Show SMILES COc1cccc(c1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-7-10-26(20-23)40-5)16-17-41-27-11-6-8-22(18-27)19-28-30(33(38)39)36(32(28)37)25-14-12-24(13-15-25)34(2,3)4/h6-15,18,20,28,30H,16-17,19H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063316
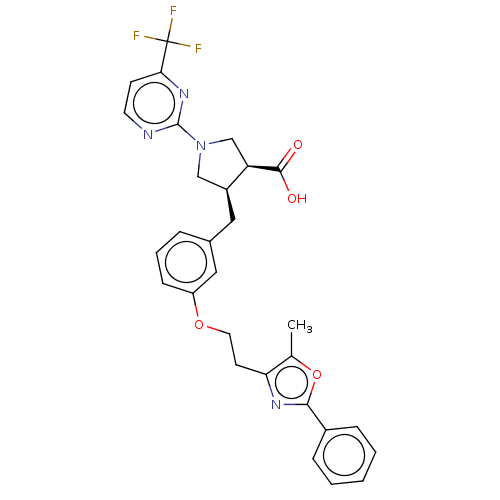
(CHEMBL3398447)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2CN(C[C@H]2C(O)=O)c2nccc(n2)C(F)(F)F)c1)-c1ccccc1 |r| Show InChI InChI=1S/C29H27F3N4O4/c1-18-24(34-26(40-18)20-7-3-2-4-8-20)11-13-39-22-9-5-6-19(15-22)14-21-16-36(17-23(21)27(37)38)28-33-12-10-25(35-28)29(30,31)32/h2-10,12,15,21,23H,11,13-14,16-17H2,1H3,(H,37,38)/t21-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50377318

(CHEMBL257345)Show SMILES CC(C)COC(=O)N1CCC(C(O)=O)=C(CCc2ccc(OCCc3nc(oc3C)-c3ccccc3)cc2)C1 |t:13| Show InChI InChI=1S/C31H36N2O6/c1-21(2)20-38-31(36)33-17-15-27(30(34)35)25(19-33)12-9-23-10-13-26(14-11-23)37-18-16-28-22(3)39-29(32-28)24-7-5-4-6-8-24/h4-8,10-11,13-14,21H,9,12,15-20H2,1-3H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Binding affinity at human PPARalpha by fluorescence polarization |
Bioorg Med Chem Lett 18: 3545-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.014
BindingDB Entry DOI: 10.7270/Q2RX9D04 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50205089

(2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...)Show SMILES Cc1oc(nc1CCOc1cccc(CN(CC(O)=O)Cc2cccc(Oc3ccccc3)c2)c1)-c1ccccc1 Show InChI InChI=1S/C34H32N2O5/c1-25-32(35-34(40-25)28-12-4-2-5-13-28)18-19-39-30-16-8-10-26(20-30)22-36(24-33(37)38)23-27-11-9-17-31(21-27)41-29-14-6-3-7-15-29/h2-17,20-21H,18-19,22-24H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50377311

(CHEMBL403432)Show SMILES CC(C)COC(=O)N1CCC(CC(O)=O)=C(C1)c1cccc(OCCc2nc(oc2C)-c2ccccc2)c1 |c:14| Show InChI InChI=1S/C30H34N2O6/c1-20(2)19-37-30(35)32-14-12-24(17-28(33)34)26(18-32)23-10-7-11-25(16-23)36-15-13-27-21(3)38-29(31-27)22-8-5-4-6-9-22/h4-11,16,20H,12-15,17-19H2,1-3H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Binding affinity at human PPARalpha by fluorescence polarization |
Bioorg Med Chem Lett 18: 3545-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.014
BindingDB Entry DOI: 10.7270/Q2RX9D04 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50377321

(CHEMBL256358)Show SMILES CC(C)COC(=O)N1CCC(C(O)=O)C(Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)=C1 |w:10.10,c:39| Show InChI InChI=1S/C30H34N2O6/c1-20(2)19-37-30(35)32-14-12-26(29(33)34)24(18-32)16-22-8-7-11-25(17-22)36-15-13-27-21(3)38-28(31-27)23-9-5-4-6-10-23/h4-11,17-18,20,26H,12-16,19H2,1-3H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Binding affinity at human PPARalpha by fluorescence polarization |
Bioorg Med Chem Lett 18: 3545-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.014
BindingDB Entry DOI: 10.7270/Q2RX9D04 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50377304

(CHEMBL401534)Show SMILES Cc1oc(nc1CCOc1cccc(CC2=CN(CCC2C(O)=O)C(=O)OCc2ccccc2)c1)-c1ccccc1 |w:20.22,t:16| Show InChI InChI=1S/C33H32N2O6/c1-23-30(34-31(41-23)26-12-6-3-7-13-26)16-18-39-28-14-8-11-25(20-28)19-27-21-35(17-15-29(27)32(36)37)33(38)40-22-24-9-4-2-5-10-24/h2-14,20-21,29H,15-19,22H2,1H3,(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Binding affinity at human PPARalpha by fluorescence polarization |
Bioorg Med Chem Lett 18: 3545-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.014
BindingDB Entry DOI: 10.7270/Q2RX9D04 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50205081

(2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...)Show SMILES Cc1oc(nc1CCOc1cccc(CN(CCc2ccccc2)CC(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C29H30N2O4/c1-22-27(30-29(35-22)25-12-6-3-7-13-25)16-18-34-26-14-8-11-24(19-26)20-31(21-28(32)33)17-15-23-9-4-2-5-10-23/h2-14,19H,15-18,20-21H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50248224

(3-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...)Show SMILES Cc1oc(nc1CCOc1cccc(Cc2nn(cc2C(O)=O)-c2ccccc2)c1)-c1ccccc1 Show InChI InChI=1S/C29H25N3O4/c1-20-26(30-28(36-20)22-10-4-2-5-11-22)15-16-35-24-14-8-9-21(17-24)18-27-25(29(33)34)19-32(31-27)23-12-6-3-7-13-23/h2-14,17,19H,15-16,18H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 1451-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.030
BindingDB Entry DOI: 10.7270/Q2MC8ZW8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data