

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50216168 (CHEMBL132778) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314731 (US9611270, Example 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314735 (US9611270, Example 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50358201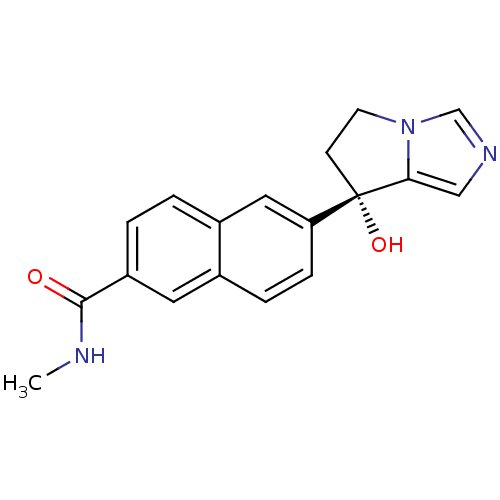 (CHEMBL1921976 | US9611270, orteronel) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50358201 (CHEMBL1921976 | US9611270, orteronel) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314733 (US9611270, Example 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50216168 (CHEMBL132778) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50456365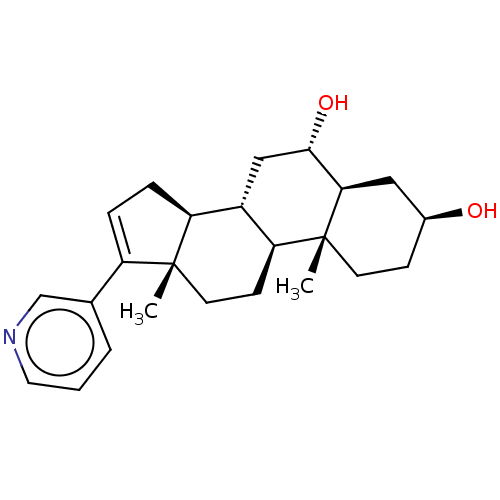 (CHEMBL4213452) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50456363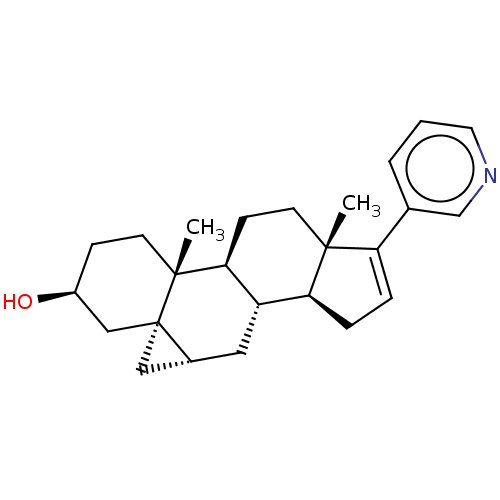 (CHEMBL4210737) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314740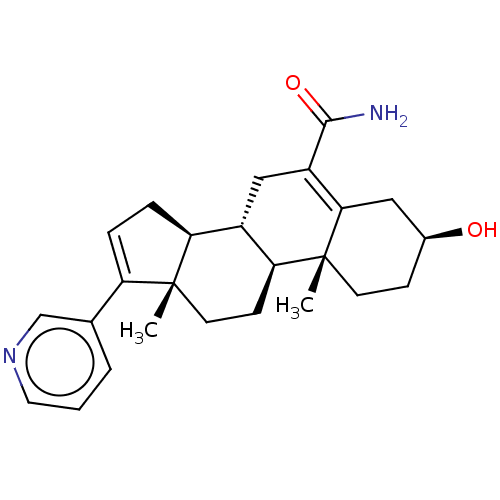 (US9611270, Example 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli JM109 cells assessed as decrease in proges... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314736 (US9611270, Example 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314732 (US9611270, Example 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314738 (US9611270, Example 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50456366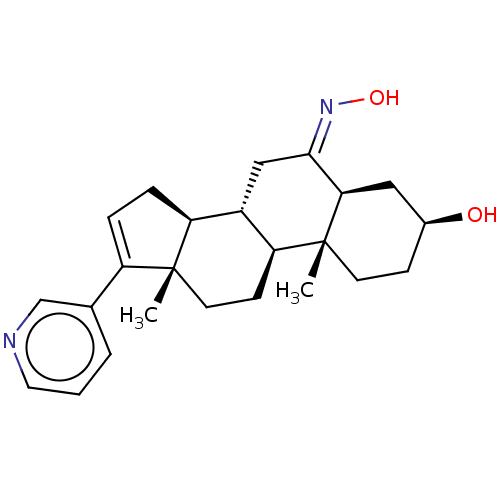 (CHEMBL4214976) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314740 (US9611270, Example 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314738 (US9611270, Example 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50435990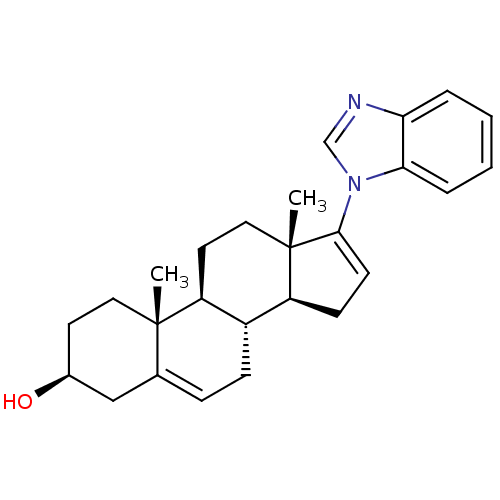 (GALETERONE | TOK-001 | US9611270, galaterone | VN/...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50456363 (CHEMBL4210737) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50435990 (GALETERONE | TOK-001 | US9611270, galaterone | VN/...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314740 (US9611270, Example 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314736 (US9611270, Example 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314738 (US9611270, Example 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314739 (US9611270, Example 39 | US9611270, Example 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314734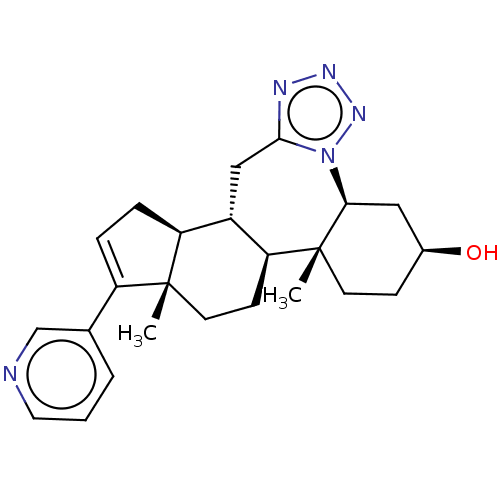 (US9611270, Example 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314732 (US9611270, Example 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP3A4 assessed as decrease in metabolism of luciferin isopropyl alcohol preincubated for 3 min... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314733 (US9611270, Example 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50456365 (CHEMBL4213452) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314737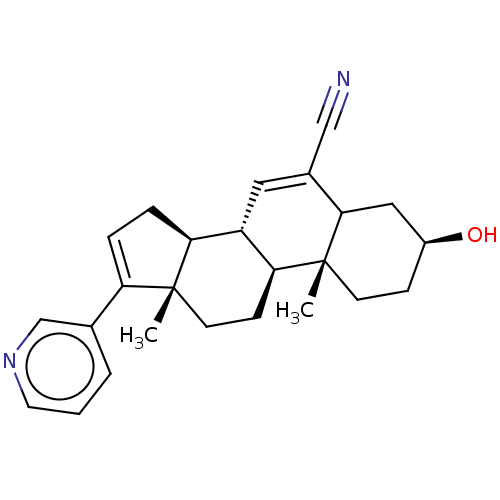 (US9611270, Example 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50435990 (GALETERONE | TOK-001 | US9611270, galaterone | VN/...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50435990 (GALETERONE | TOK-001 | US9611270, galaterone | VN/...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314730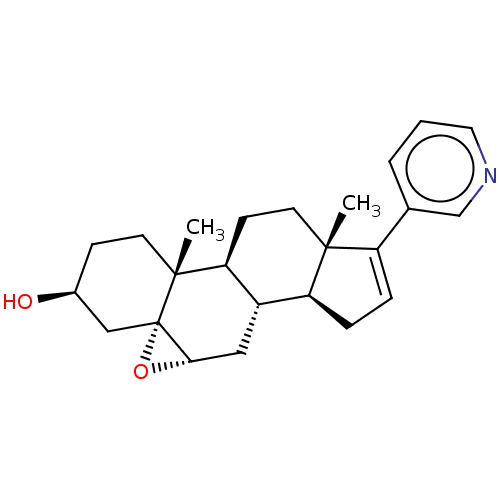 (US9611270, Example 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 428 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314732 (US9611270, Example 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314738 (US9611270, Example 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314740 (US9611270, Example 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 673 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314739 (US9611270, Example 39 | US9611270, Example 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 831 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50456366 (CHEMBL4214976) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314733 (US9611270, Example 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50456366 (CHEMBL4214976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP3A4 assessed as decrease in metabolism of luciferin isopropyl alcohol preincubated for 3 min... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50456364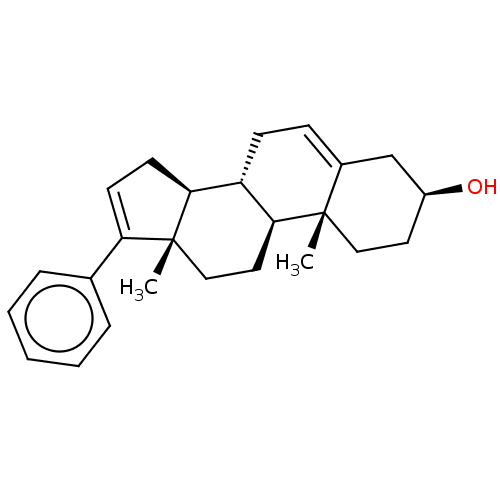 (CHEMBL4203137) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli DH5alpha assessed as decrease in progester... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||