 Found 160 hits with Last Name = 'feixas' and Initial = 'j'
Found 160 hits with Last Name = 'feixas' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50123473
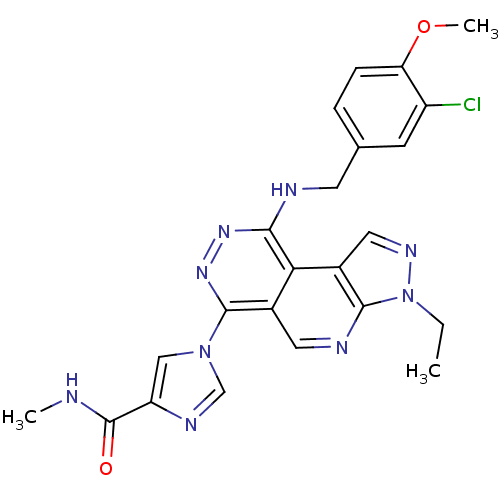
(1-[9-(3-Chloro-4-methoxy-benzylamino)-3-ethyl-3H-2...)Show SMILES CCn1ncc2c1ncc1c(nnc(NCc3ccc(OC)c(Cl)c3)c21)-n1cnc(c1)C(=O)NC Show InChI InChI=1S/C23H22ClN9O2/c1-4-33-21-15(10-29-33)19-14(9-27-21)22(32-11-17(28-12-32)23(34)25-2)31-30-20(19)26-8-13-5-6-18(35-3)16(24)7-13/h5-7,9-12H,4,8H2,1-3H3,(H,25,34)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5; IC50 range 0.03-0.3 nM |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165805
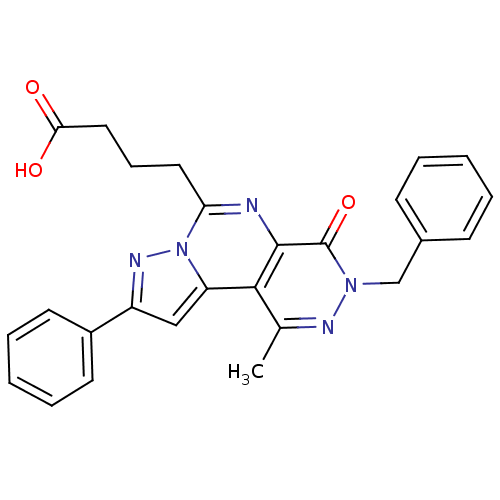
(4-(7-Benzyl-9-methyl-6-oxo-2-phenyl-6,7-dihydro-3,...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(CCCC(O)=O)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C26H23N5O3/c1-17-24-21-15-20(19-11-6-3-7-12-19)29-31(21)22(13-8-14-23(32)33)27-25(24)26(34)30(28-17)16-18-9-4-2-5-10-18/h2-7,9-12,15H,8,13-14,16H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165811

(7-Benzyl-4-sec-butyl-9-methyl-2-phenyl-7H-3,3a,5,7...)Show SMILES CCC(C)c1nc2c(c(C)nn(Cc3ccccc3)c2=O)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C26H25N5O/c1-4-17(2)25-27-24-23(22-15-21(29-31(22)25)20-13-9-6-10-14-20)18(3)28-30(26(24)32)16-19-11-7-5-8-12-19/h5-15,17H,4,16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165812
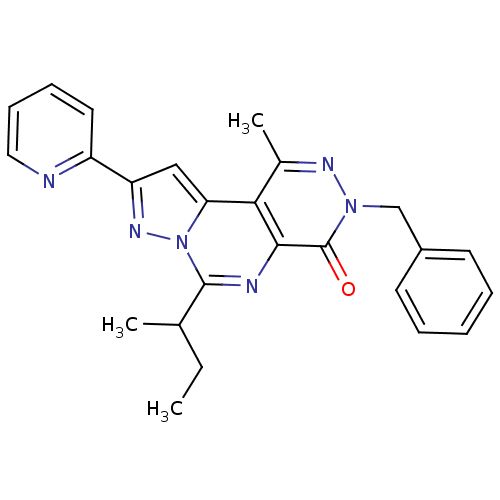
(7-Benzyl-4-sec-butyl-9-methyl-2-pyridin-2-yl-7H-3,...)Show SMILES CCC(C)c1nc2c(c(C)nn(Cc3ccccc3)c2=O)c2cc(nn12)-c1ccccn1 Show InChI InChI=1S/C25H24N6O/c1-4-16(2)24-27-23-22(21-14-20(29-31(21)24)19-12-8-9-13-26-19)17(3)28-30(25(23)32)15-18-10-6-5-7-11-18/h5-14,16H,4,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of bovine Phosphodiesterase 6 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165826

(CHEMBL193746 | Phosphoric acid mono-[3-(4-sec-buty...)Show SMILES CCC(C)c1nc2c(c(C)nn(Cc3cccc(OP(O)(O)=O)c3)c2=O)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C26H26N5O5P/c1-4-16(2)25-27-24-23(22-14-21(29-31(22)25)19-10-6-5-7-11-19)17(3)28-30(26(24)32)15-18-9-8-12-20(13-18)36-37(33,34)35/h5-14,16H,4,15H2,1-3H3,(H2,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50165810
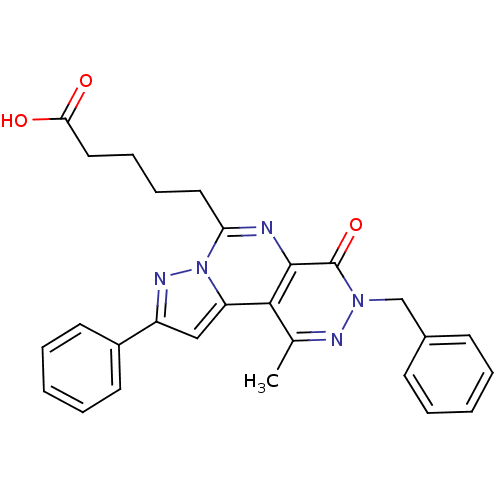
(5-(7-Benzyl-9-methyl-6-oxo-2-phenyl-6,7-dihydro-3,...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(CCCCC(O)=O)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C27H25N5O3/c1-18-25-22-16-21(20-12-6-3-7-13-20)30-32(22)23(14-8-9-15-24(33)34)28-26(25)27(35)31(29-18)17-19-10-4-2-5-11-19/h2-7,10-13,16H,8-9,14-15,17H2,1H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of bovine Phosphodiesterase 6 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165822
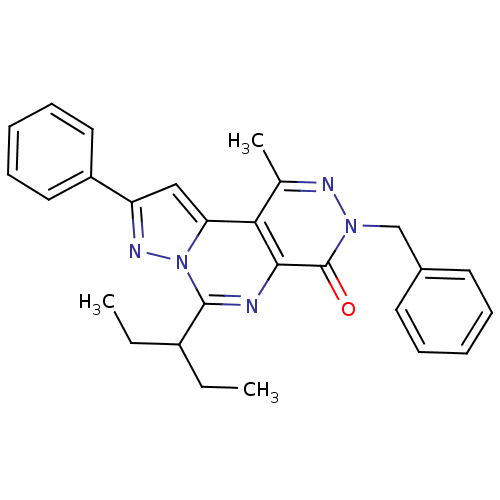
(7-Benzyl-4-(1-ethyl-propyl)-9-methyl-2-phenyl-7H-3...)Show SMILES CCC(CC)c1nc2c(c(C)nn(Cc3ccccc3)c2=O)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C27H27N5O/c1-4-20(5-2)26-28-25-24(23-16-22(30-32(23)26)21-14-10-7-11-15-21)18(3)29-31(27(25)33)17-19-12-8-6-9-13-19/h6-16,20H,4-5,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165818
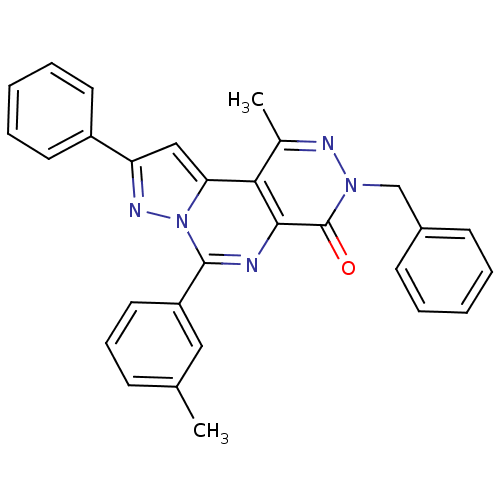
(7-Benzyl-9-methyl-2-phenyl-4-m-tolyl-7H-3,3a,5,7,8...)Show SMILES Cc1cccc(c1)-c1nc2c(c(C)nn(Cc3ccccc3)c2=O)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C29H23N5O/c1-19-10-9-15-23(16-19)28-30-27-26(25-17-24(32-34(25)28)22-13-7-4-8-14-22)20(2)31-33(29(27)35)18-21-11-5-3-6-12-21/h3-17H,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165806

(7-Benzyl-9-methyl-2,4-diphenyl-7H-3,3a,5,7,8-penta...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(-c3ccccc3)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C28H21N5O/c1-19-25-24-17-23(21-13-7-3-8-14-21)31-33(24)27(22-15-9-4-10-16-22)29-26(25)28(34)32(30-19)18-20-11-5-2-6-12-20/h2-17H,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165814

(7-Benzyl-9-methyl-2-phenyl-4-pyridin-2-yl-7H-3,3a,...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(-c3ccccn3)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C27H20N6O/c1-18-24-23-16-22(20-12-6-3-7-13-20)31-33(23)26(21-14-8-9-15-28-21)29-25(24)27(34)32(30-18)17-19-10-4-2-5-11-19/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165810

(5-(7-Benzyl-9-methyl-6-oxo-2-phenyl-6,7-dihydro-3,...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(CCCCC(O)=O)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C27H25N5O3/c1-18-25-22-16-21(20-12-6-3-7-13-20)30-32(22)23(14-8-9-15-24(33)34)28-26(25)27(35)31(29-18)17-19-10-4-2-5-11-19/h2-7,10-13,16H,8-9,14-15,17H2,1H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165828

(7-Benzyl-9-methyl-4-methylsulfanylmethyl-2-phenyl-...)Show SMILES CSCc1nc2c(c(C)nn(Cc3ccccc3)c2=O)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C24H21N5OS/c1-16-22-20-13-19(18-11-7-4-8-12-18)27-29(20)21(15-31-2)25-23(22)24(30)28(26-16)14-17-9-5-3-6-10-17/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165827

(7-Benzyl-4-methoxymethyl-9-methyl-2-phenyl-7H-3,3a...)Show SMILES COCc1nc2c(c(C)nn(Cc3ccccc3)c2=O)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C24H21N5O2/c1-16-22-20-13-19(18-11-7-4-8-12-18)27-29(20)21(15-31-2)25-23(22)24(30)28(26-16)14-17-9-5-3-6-10-17/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50165805

(4-(7-Benzyl-9-methyl-6-oxo-2-phenyl-6,7-dihydro-3,...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(CCCC(O)=O)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C26H23N5O3/c1-17-24-21-15-20(19-11-6-3-7-12-19)29-31(21)22(13-8-14-23(32)33)27-25(24)26(34)30(28-17)16-18-9-4-2-5-10-18/h2-7,9-12,15H,8,13-14,16H2,1H3,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of bovine Phosphodiesterase 6 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165819

(7-Benzyl-4,9-dimethyl-2-phenyl-7H-3,3a,5,7,8-penta...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(C)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C23H19N5O/c1-15-21-20-13-19(18-11-7-4-8-12-18)26-28(20)16(2)24-22(21)23(29)27(25-15)14-17-9-5-3-6-10-17/h3-13H,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165808
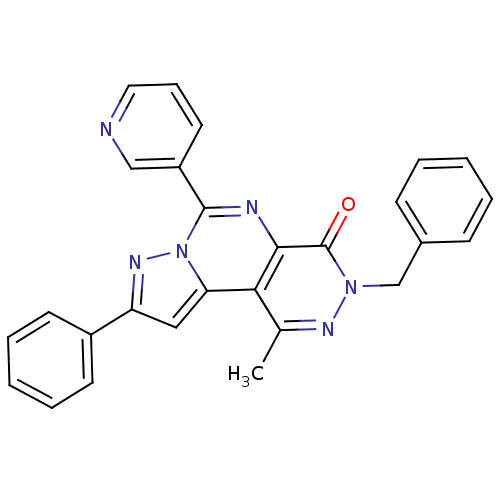
(7-Benzyl-9-methyl-2-phenyl-4-pyridin-3-yl-7H-3,3a,...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(-c3cccnc3)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C27H20N6O/c1-18-24-23-15-22(20-11-6-3-7-12-20)31-33(23)26(21-13-8-14-28-16-21)29-25(24)27(34)32(30-18)17-19-9-4-2-5-10-19/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084355
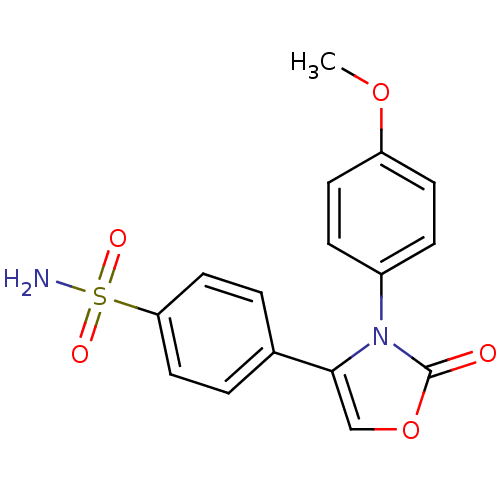
(4-[3-(4-Methoxy-phenyl)-2-oxo-2,3-dihydro-oxazol-4...)Show SMILES COc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O5S/c1-22-13-6-4-12(5-7-13)18-15(10-23-16(18)19)11-2-8-14(9-3-11)24(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105290

(2-[7-Methoxy-4-(4-methyl-benzyl)-naphthalen-1-yl]-...)Show InChI InChI=1S/C22H22O3/c1-14-4-6-16(7-5-14)12-17-8-10-19(15(2)22(23)24)21-13-18(25-3)9-11-20(17)21/h4-11,13,15H,12H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165825
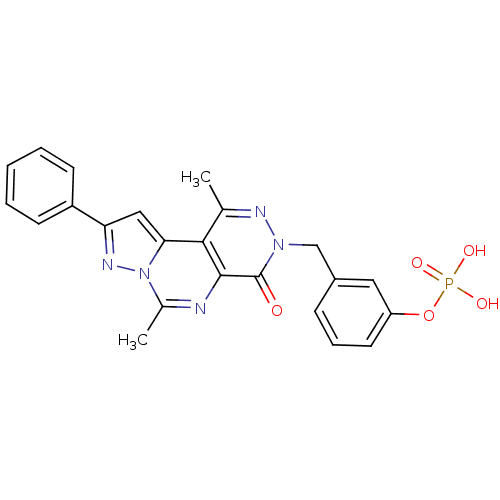
(CHEMBL363638 | Phosphoric acid mono-[3-(4,9-dimeth...)Show SMILES Cc1nn(Cc2cccc(OP(O)(O)=O)c2)c(=O)c2nc(C)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C23H20N5O5P/c1-14-21-20-12-19(17-8-4-3-5-9-17)26-28(20)15(2)24-22(21)23(29)27(25-14)13-16-7-6-10-18(11-16)33-34(30,31)32/h3-12H,13H2,1-2H3,(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084352

(4-[3-(3,4-Dichloro-phenyl)-5-methyl-2-oxo-2,3-dihy...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C16H12Cl2N2O4S/c1-9-15(10-2-5-12(6-3-10)25(19,22)23)20(16(21)24-9)11-4-7-13(17)14(18)8-11/h2-8H,1H3,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165815
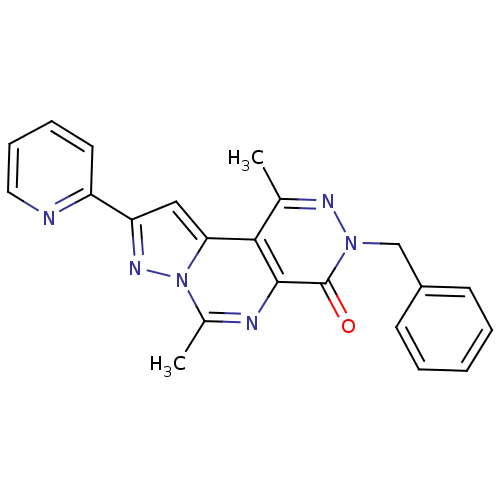
(7-Benzyl-4,9-dimethyl-2-pyridin-2-yl-7H-3,3a,5,7,8...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(C)n3nc(cc3c12)-c1ccccn1 Show InChI InChI=1S/C22H18N6O/c1-14-20-19-12-18(17-10-6-7-11-23-17)26-28(19)15(2)24-21(20)22(29)27(25-14)13-16-8-4-3-5-9-16/h3-12H,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084366
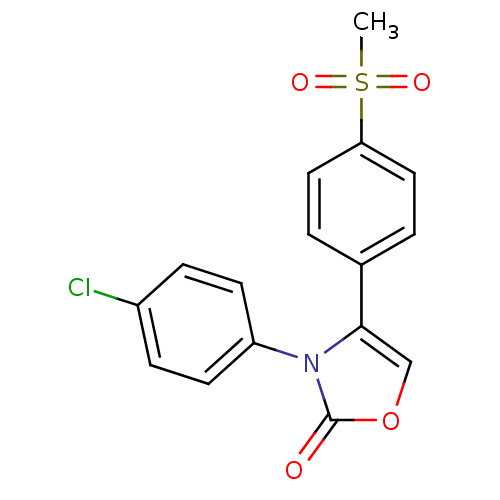
(3-(4-Chloro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C16H12ClNO4S/c1-23(20,21)14-8-2-11(3-9-14)15-10-22-16(19)18(15)13-6-4-12(17)5-7-13/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165809

((E)-3-(7-Benzyl-9-methyl-6-oxo-2-phenyl-6,7-dihydr...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(\C=C\C(O)=O)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C25H19N5O3/c1-16-23-20-14-19(18-10-6-3-7-11-18)28-30(20)21(12-13-22(31)32)26-24(23)25(33)29(27-16)15-17-8-4-2-5-9-17/h2-14H,15H2,1H3,(H,31,32)/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105302
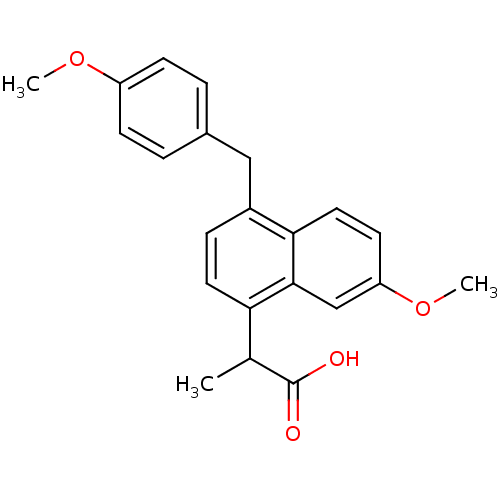
(2-[7-Methoxy-4-(4-methoxy-benzyl)-naphthalen-1-yl]...)Show SMILES COc1ccc(Cc2ccc(C(C)C(O)=O)c3cc(OC)ccc23)cc1 Show InChI InChI=1S/C22H22O4/c1-14(22(23)24)19-10-6-16(12-15-4-7-17(25-2)8-5-15)20-11-9-18(26-3)13-21(19)20/h4-11,13-14H,12H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165823
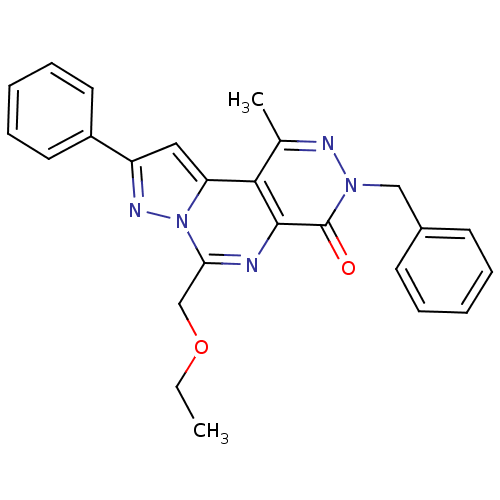
(7-Benzyl-4-ethoxymethyl-9-methyl-2-phenyl-7H-3,3a,...)Show SMILES CCOCc1nc2c(c(C)nn(Cc3ccccc3)c2=O)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C25H23N5O2/c1-3-32-16-22-26-24-23(21-14-20(28-30(21)22)19-12-8-5-9-13-19)17(2)27-29(25(24)31)15-18-10-6-4-7-11-18/h4-14H,3,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105293
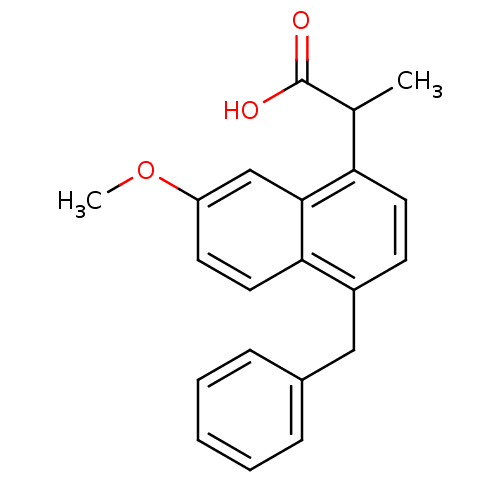
(2-(4-Benzyl-7-methoxy-naphthalen-1-yl)-propionic a...)Show InChI InChI=1S/C21H20O3/c1-14(21(22)23)18-10-8-16(12-15-6-4-3-5-7-15)19-11-9-17(24-2)13-20(18)19/h3-11,13-14H,12H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084359
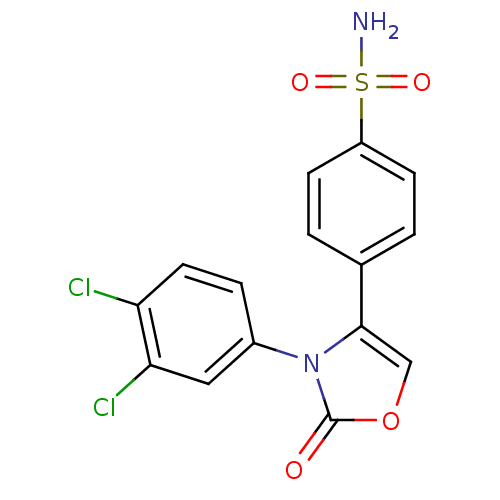
(4-[3-(3,4-Dichloro-phenyl)-2-oxo-2,3-dihydro-oxazo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H10Cl2N2O4S/c16-12-6-3-10(7-13(12)17)19-14(8-23-15(19)20)9-1-4-11(5-2-9)24(18,21)22/h1-8H,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165816

(3-(7-Benzyl-9-methyl-6-oxo-2-phenyl-6,7-dihydro-3,...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(CCC(O)=O)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C25H21N5O3/c1-16-23-20-14-19(18-10-6-3-7-11-18)28-30(20)21(12-13-22(31)32)26-24(23)25(33)29(27-16)15-17-8-4-2-5-9-17/h2-11,14H,12-13,15H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165817
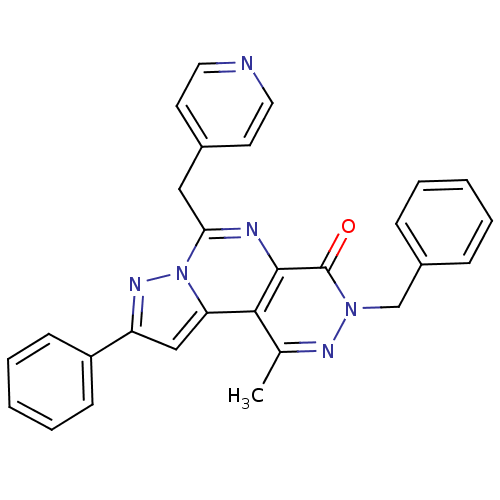
(7-Benzyl-9-methyl-2-phenyl-4-pyridin-4-ylmethyl-7H...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(Cc3ccncc3)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C28H22N6O/c1-19-26-24-17-23(22-10-6-3-7-11-22)32-34(24)25(16-20-12-14-29-15-13-20)30-27(26)28(35)33(31-19)18-21-8-4-2-5-9-21/h2-15,17H,16,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 5 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084345
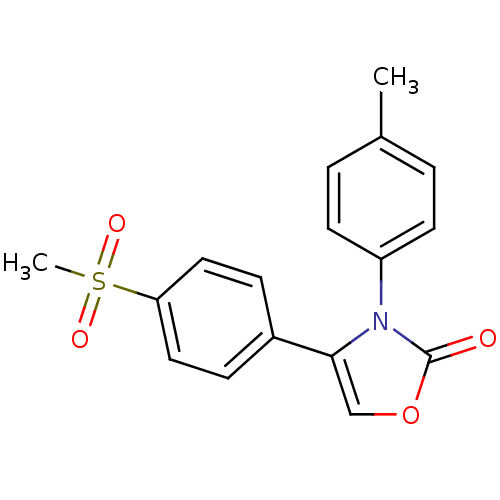
(4-(4-Methanesulfonyl-phenyl)-3-p-tolyl-3H-oxazol-2...)Show SMILES Cc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C17H15NO4S/c1-12-3-7-14(8-4-12)18-16(11-22-17(18)19)13-5-9-15(10-6-13)23(2,20)21/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084347
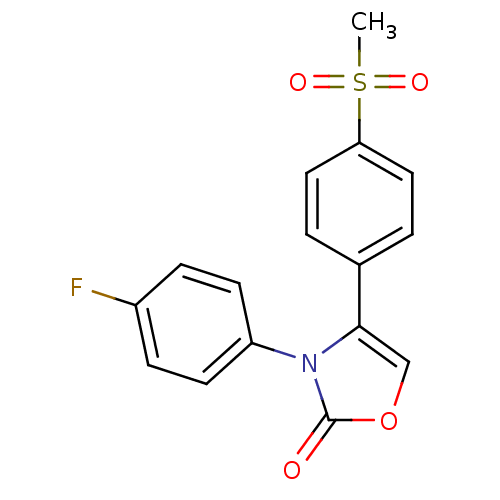
(3-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C16H12FNO4S/c1-23(20,21)14-8-2-11(3-9-14)15-10-22-16(19)18(15)13-6-4-12(17)5-7-13/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084368
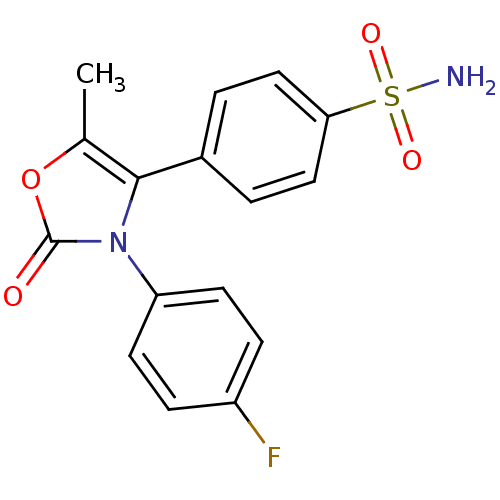
(4-[3-(4-Fluoro-phenyl)-5-methyl-2-oxo-2,3-dihydro-...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C16H13FN2O4S/c1-10-15(11-2-8-14(9-3-11)24(18,21)22)19(16(20)23-10)13-6-4-12(17)5-7-13/h2-9H,1H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084353
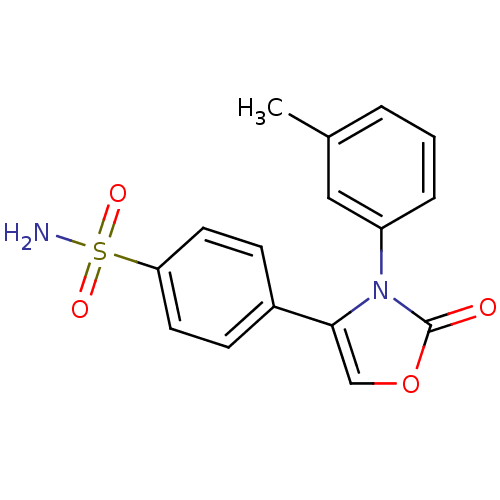
(4-(2-Oxo-3-m-tolyl-2,3-dihydro-oxazol-4-yl)-benzen...)Show SMILES Cc1cccc(c1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O4S/c1-11-3-2-4-13(9-11)18-15(10-22-16(18)19)12-5-7-14(8-6-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105303
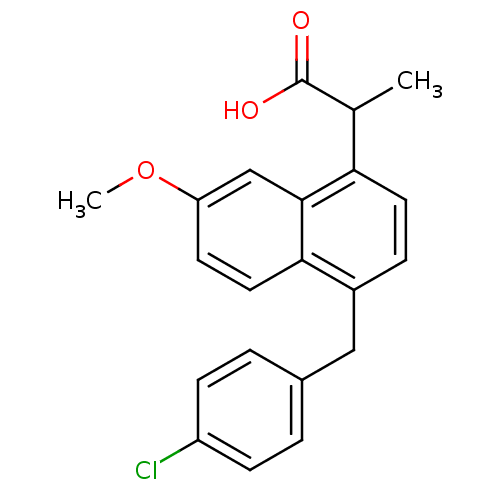
(2-[4-(4-Chloro-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show SMILES COc1ccc2c(Cc3ccc(Cl)cc3)ccc(C(C)C(O)=O)c2c1 Show InChI InChI=1S/C21H19ClO3/c1-13(21(23)24)18-9-5-15(11-14-3-6-16(22)7-4-14)19-10-8-17(25-2)12-20(18)19/h3-10,12-13H,11H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50165809

((E)-3-(7-Benzyl-9-methyl-6-oxo-2-phenyl-6,7-dihydr...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(\C=C\C(O)=O)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C25H19N5O3/c1-16-23-20-14-19(18-10-6-3-7-11-18)28-30(20)21(12-13-22(31)32)26-24(23)25(33)29(27-16)15-17-8-4-2-5-9-17/h2-14H,15H2,1H3,(H,31,32)/b13-12+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of bovine Phosphodiesterase 6 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50165816

(3-(7-Benzyl-9-methyl-6-oxo-2-phenyl-6,7-dihydro-3,...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2nc(CCC(O)=O)n3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C25H21N5O3/c1-16-23-20-14-19(18-10-6-3-7-11-18)28-30(20)21(12-13-22(31)32)26-24(23)25(33)29(27-16)15-17-8-4-2-5-9-17/h2-11,14H,12-13,15H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of bovine Phosphodiesterase 6 (n=2-3) |
Bioorg Med Chem Lett 15: 2381-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.090
BindingDB Entry DOI: 10.7270/Q2K64HK9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084349
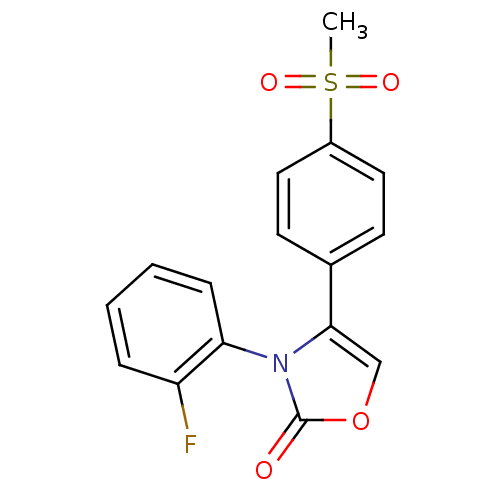
(3-(2-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccccc1F |(5.04,-8.06,;6.08,-9.19,;7.18,-10.29,;5.08,-10.45,;7.11,-8.04,;6.63,-6.59,;7.67,-5.45,;9.16,-5.76,;9.64,-7.22,;8.62,-8.36,;10.18,-4.61,;11.71,-4.76,;12.32,-3.35,;11.18,-2.33,;11.34,-.8,;9.85,-3.1,;8.76,-2.01,;9.16,-.51,;8.06,.6,;6.55,.19,;6.15,-1.33,;7.26,-2.42,;6.87,-3.89,)| Show InChI InChI=1S/C16H12FNO4S/c1-23(20,21)12-8-6-11(7-9-12)15-10-22-16(19)18(15)14-5-3-2-4-13(14)17/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084346
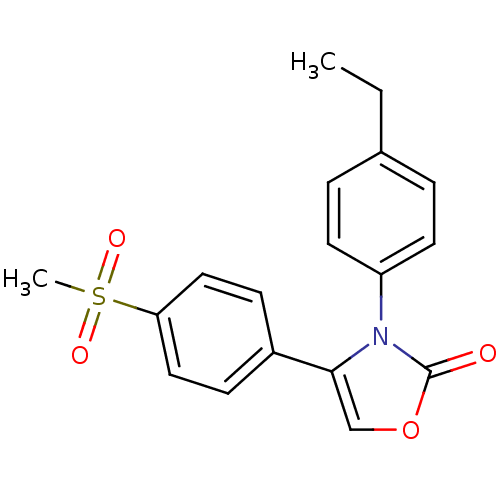
(3-(4-Ethyl-phenyl)-4-(4-methanesulfonyl-phenyl)-3H...)Show SMILES CCc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H17NO4S/c1-3-13-4-8-15(9-5-13)19-17(12-23-18(19)20)14-6-10-16(11-7-14)24(2,21)22/h4-12H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105289
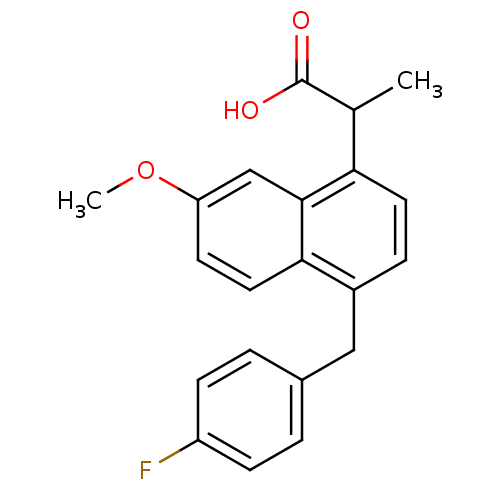
(2-[4-(4-Fluoro-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show InChI InChI=1S/C21H19FO3/c1-13(21(23)24)18-9-5-15(11-14-3-6-16(22)7-4-14)19-10-8-17(25-2)12-20(18)19/h3-10,12-13H,11H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084370

(4-[3-(3-Chloro-4-methoxy-phenyl)-2-oxo-2,3-dihydro...)Show SMILES COc1ccc(cc1Cl)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13ClN2O5S/c1-23-15-7-4-11(8-13(15)17)19-14(9-24-16(19)20)10-2-5-12(6-3-10)25(18,21)22/h2-9H,1H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084364
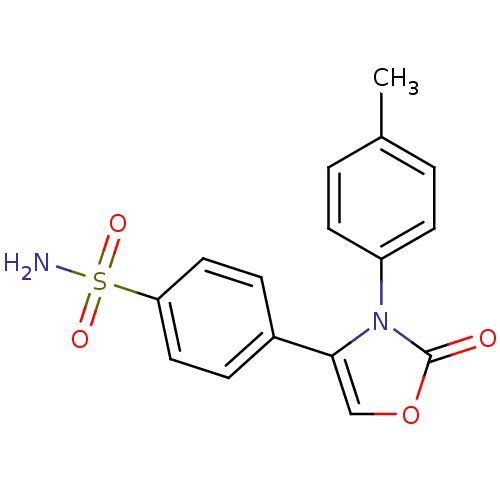
(4-(2-Oxo-3-p-tolyl-2,3-dihydro-oxazol-4-yl)-benzen...)Show SMILES Cc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O4S/c1-11-2-6-13(7-3-11)18-15(10-22-16(18)19)12-4-8-14(9-5-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105288
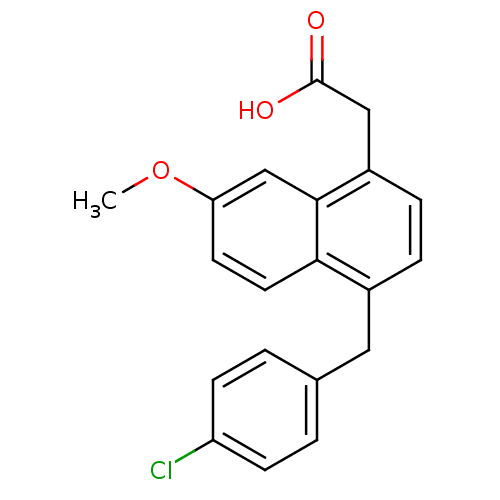
(CHEMBL91139 | [4-(4-Chloro-benzyl)-7-methoxy-napht...)Show InChI InChI=1S/C20H17ClO3/c1-24-17-8-9-18-14(10-13-2-6-16(21)7-3-13)4-5-15(11-20(22)23)19(18)12-17/h2-9,12H,10-11H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084355

(4-[3-(4-Methoxy-phenyl)-2-oxo-2,3-dihydro-oxazol-4...)Show SMILES COc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O5S/c1-22-13-6-4-12(5-7-13)18-15(10-23-16(18)19)11-2-8-14(9-3-11)24(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data