

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute Curated by ChEMBL | Assay Description Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G | Bioorg Med Chem Lett 20: 7222-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.109 BindingDB Entry DOI: 10.7270/Q2GB24B9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50322367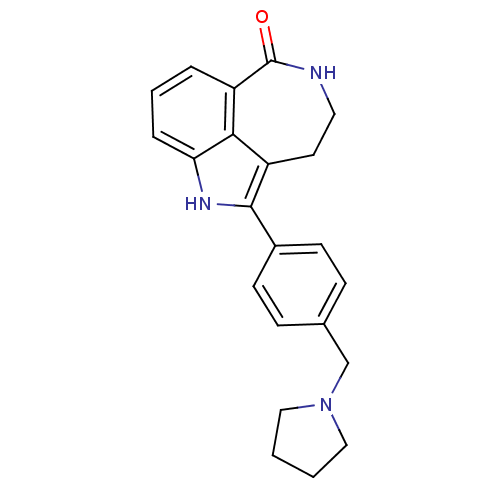 (5-(4-(pyrrolidin-1-ylmethyl)phenyl)-2,3,4,6-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50154730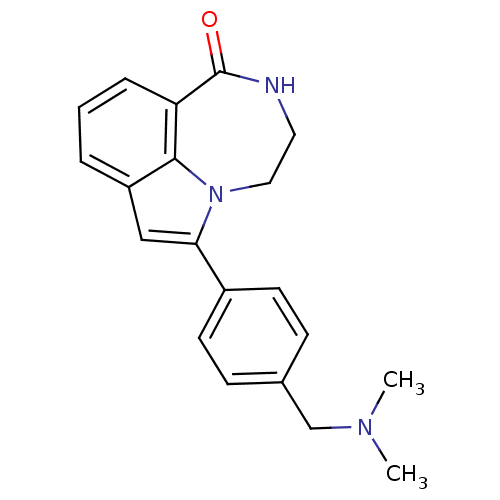 (6-(4-((dimethylamino)methyl)phenyl)-3,4-dihydro-[1...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50117763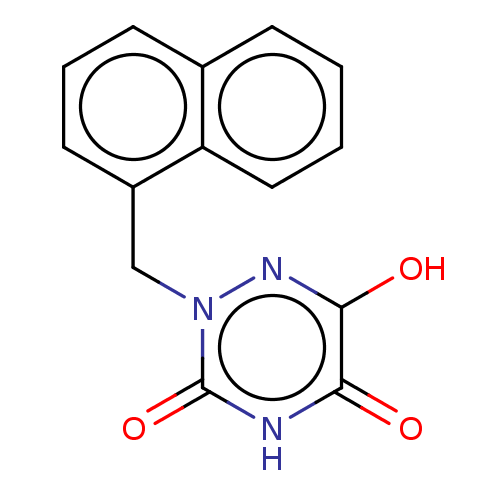 (CHEMBL3613921 | US9505753, 5u) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human DAAO expressed in HEK cells by double reciprocal plot analysis in presence of D-serine | J Med Chem 58: 7258-72 (2015) Article DOI: 10.1021/acs.jmedchem.5b00482 BindingDB Entry DOI: 10.7270/Q2SF2XZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase family 1 member A3 (Homo sapiens (Human)) | BDBM50538688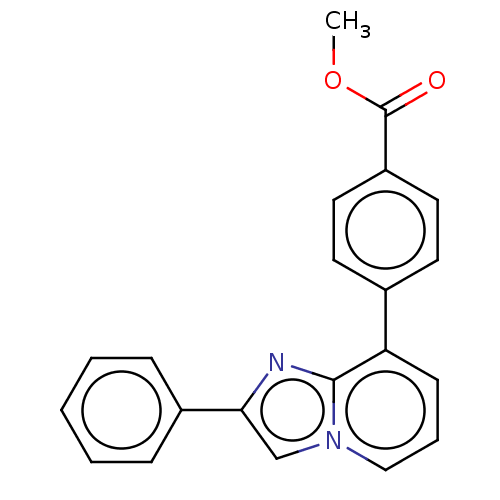 (CHEMBL4642789) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ALDH1A3 pre-incubated for 5 mins before acetaldehyde addition by continuous spectrometric assay relative ... | ACS Med Chem Lett 11: 963-970 (2020) Article DOI: 10.1021/acsmedchemlett.9b00686 BindingDB Entry DOI: 10.7270/Q2N301G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase family 1 member A3 (Homo sapiens (Human)) | BDBM50527781 (CHEMBL4456676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human 6His-tagged ALDH1A3 expressed in Escherichia coli BL21(DE3) using varying level of acetaldehyde as substr... | J Med Chem 63: 4603-4616 (2020) Article DOI: 10.1021/acs.jmedchem.9b01910 BindingDB Entry DOI: 10.7270/Q22V2KJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinal dehydrogenase 2 (Homo sapiens (Human)) | BDBM50538688 (CHEMBL4642789) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ALDH1A2 pre-incubated for 5 mins before acetaldehyde addition by continuous spectrometric assay relative ... | ACS Med Chem Lett 11: 963-970 (2020) Article DOI: 10.1021/acsmedchemlett.9b00686 BindingDB Entry DOI: 10.7270/Q2N301G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50456068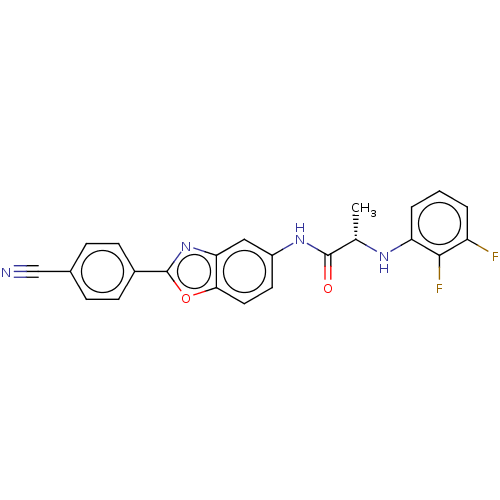 (CHEMBL4208344) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis IMPDH2 Y487C mutant using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456074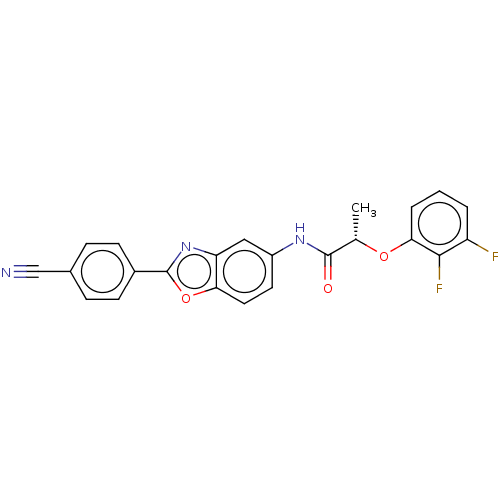 (CHEMBL4202438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM108460 (CHEMBL2178393 | US11191732, Example 1 | US8604016,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysis | J Med Chem 55: 10551-63 (2012) Article DOI: 10.1021/jm301191p BindingDB Entry DOI: 10.7270/Q2VD70M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456086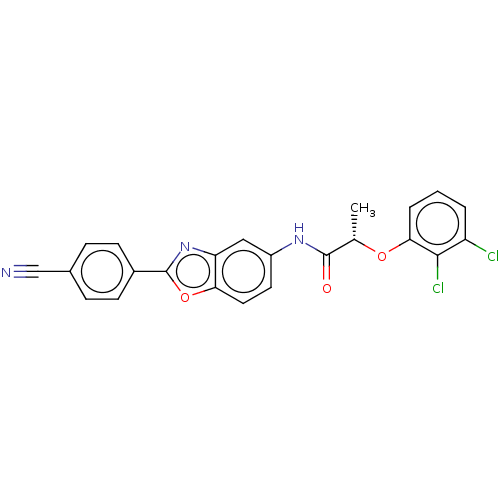 (CHEMBL4204706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456092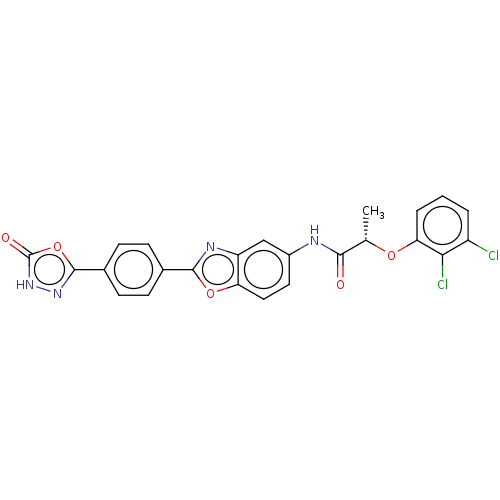 (CHEMBL4218122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456097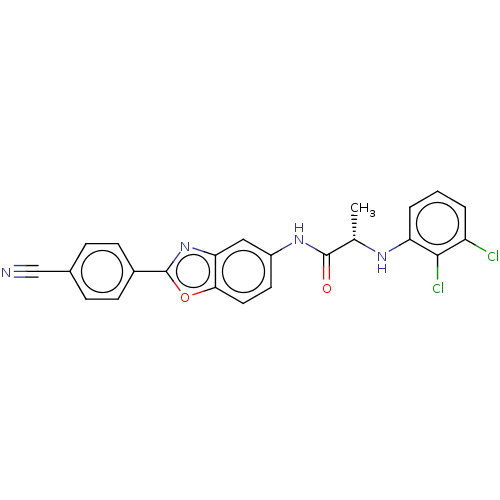 (CHEMBL4217858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456098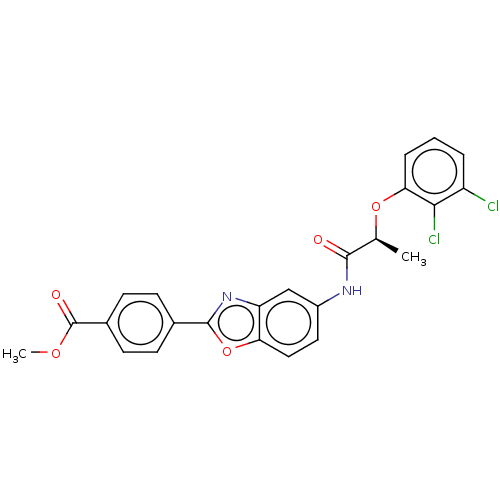 (CHEMBL4214376) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456089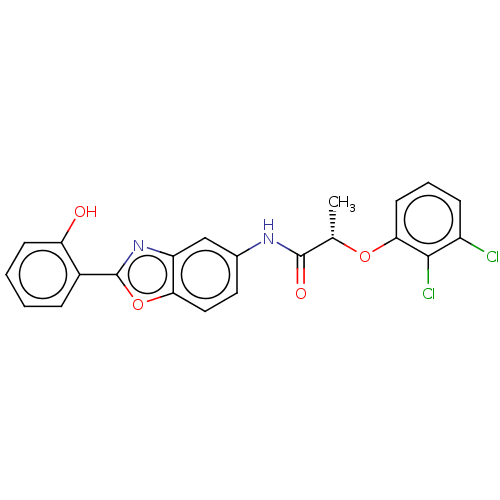 (CHEMBL4205578) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456082 (CHEMBL4211507) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456099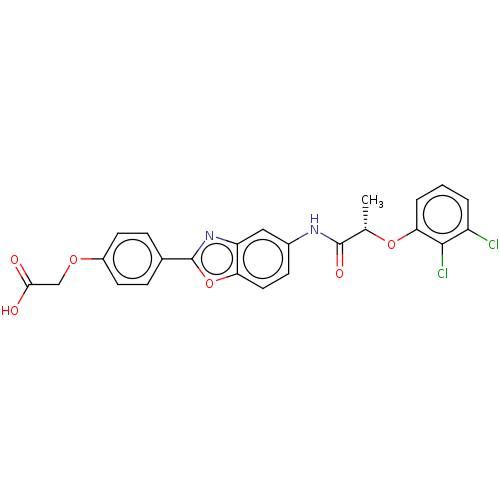 (CHEMBL4215689) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456090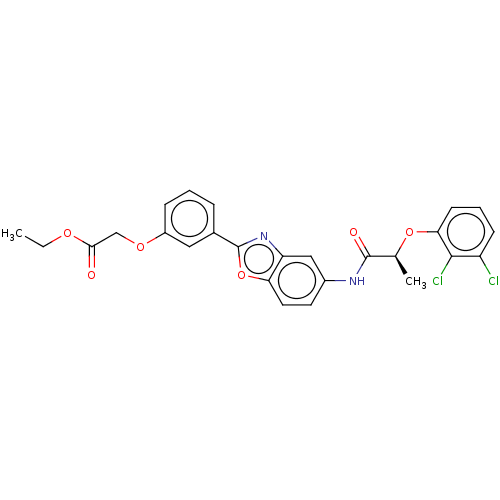 (CHEMBL4210733) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456091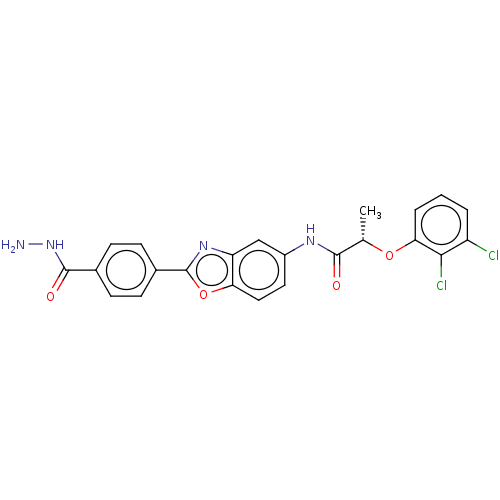 (CHEMBL4218662) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456067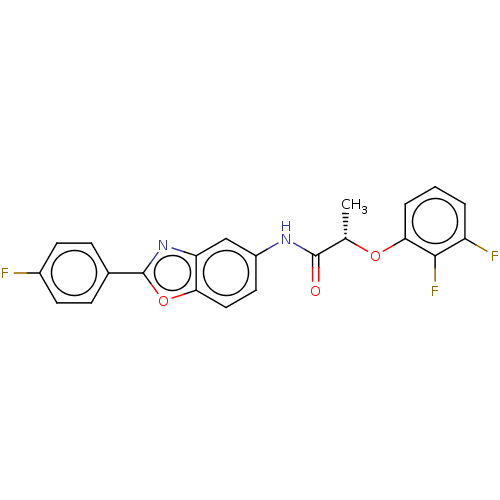 (CHEMBL4203696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456079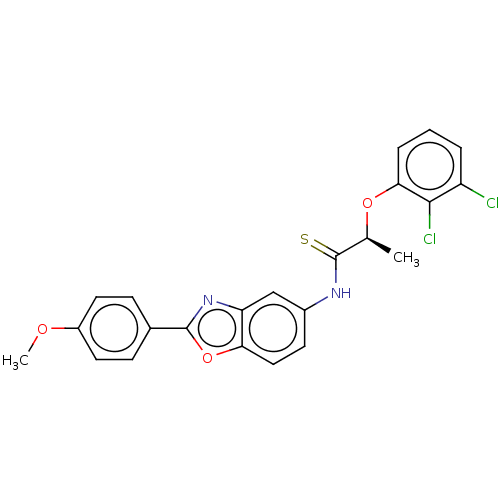 (CHEMBL4209344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456100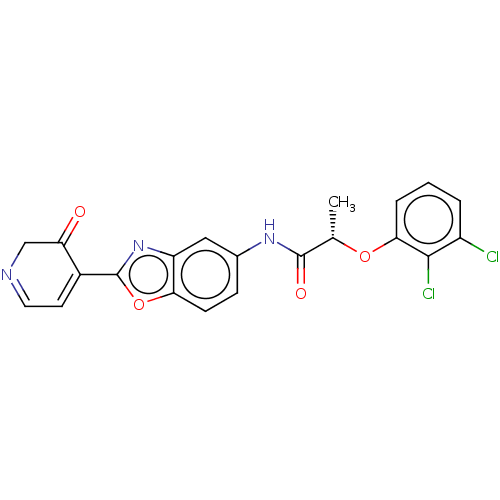 (CHEMBL4209101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456101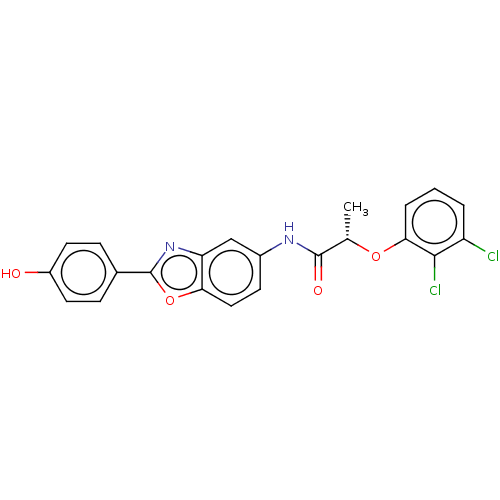 (CHEMBL4213736) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456083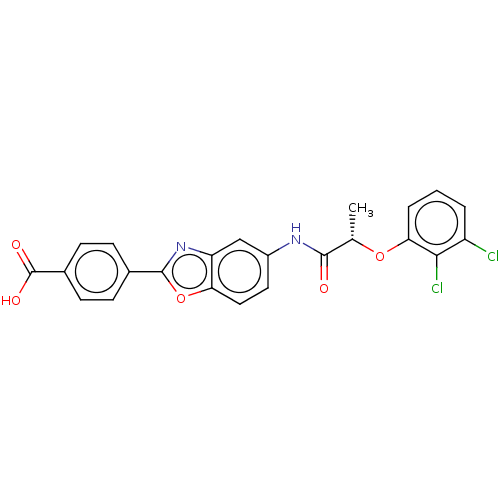 (CHEMBL4213733) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456093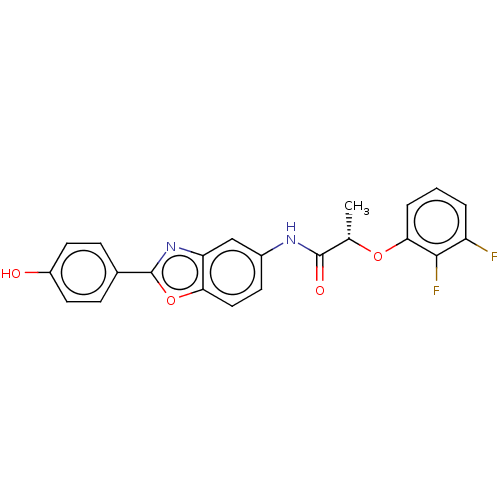 (CHEMBL4213588) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456102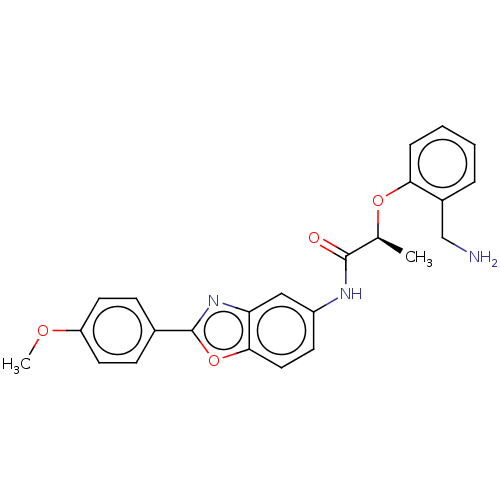 (CHEMBL4207404) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456100 (CHEMBL4209101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456098 (CHEMBL4214376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456103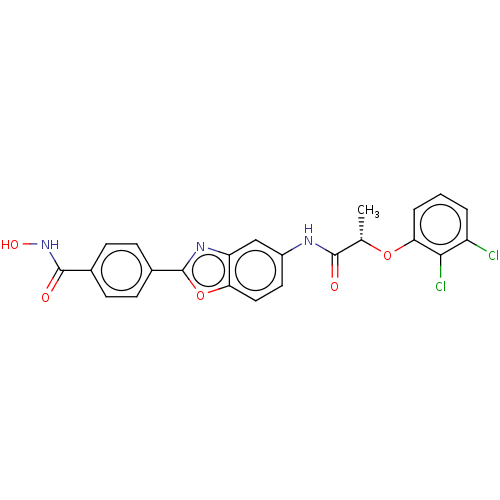 (CHEMBL4207011) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456104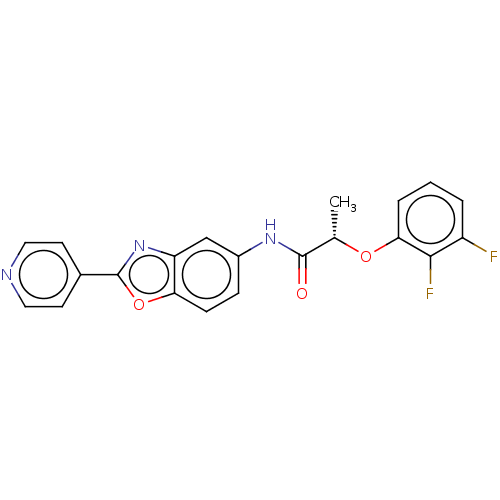 (CHEMBL4209137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456105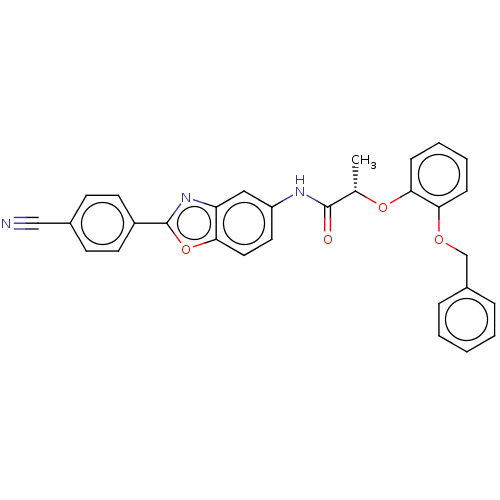 (CHEMBL4212299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50432798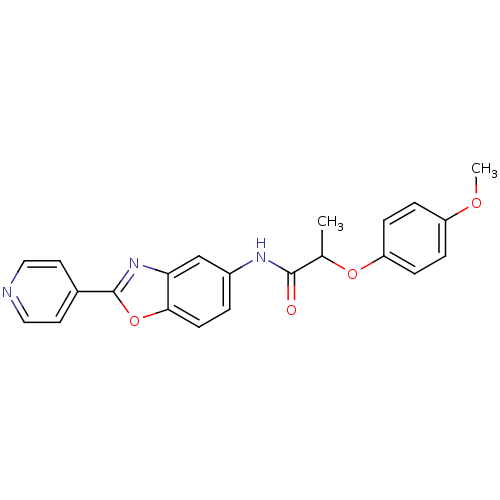 (CHEMBL2348814) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456087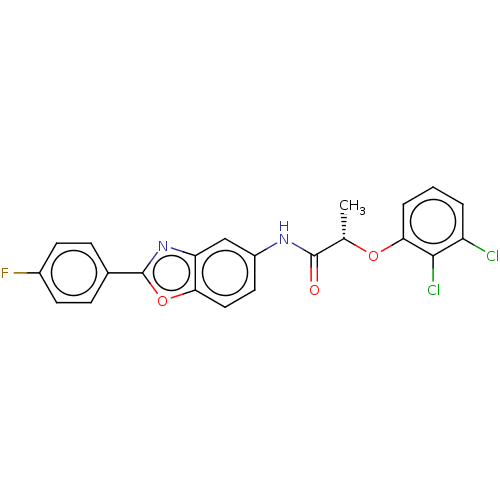 (CHEMBL4208384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456103 (CHEMBL4207011) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456104 (CHEMBL4209137) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456105 (CHEMBL4212299) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456078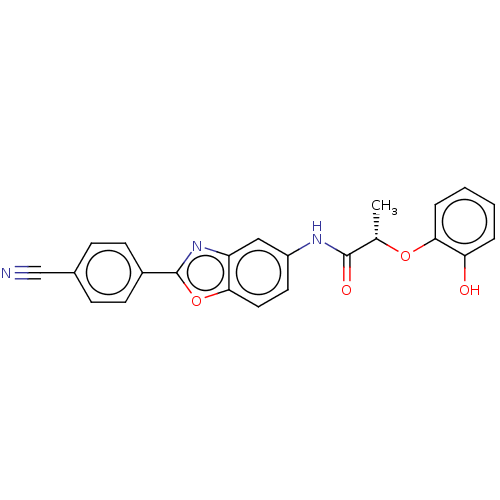 (CHEMBL4207934) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456097 (CHEMBL4217858) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50432765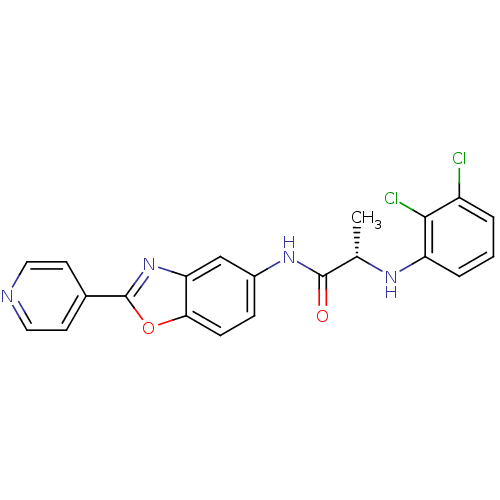 (CHEMBL2348794) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456106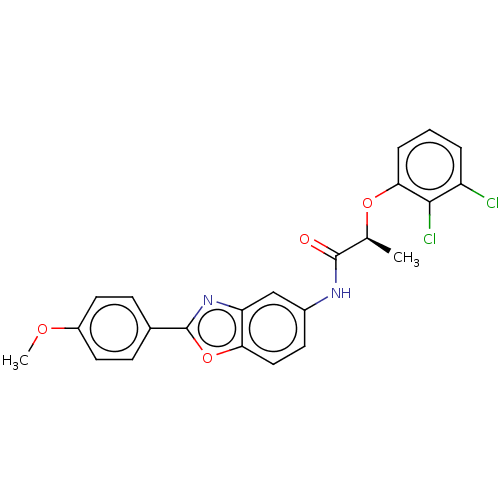 (CHEMBL4207207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456101 (CHEMBL4213736) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456099 (CHEMBL4215689) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456083 (CHEMBL4213733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456070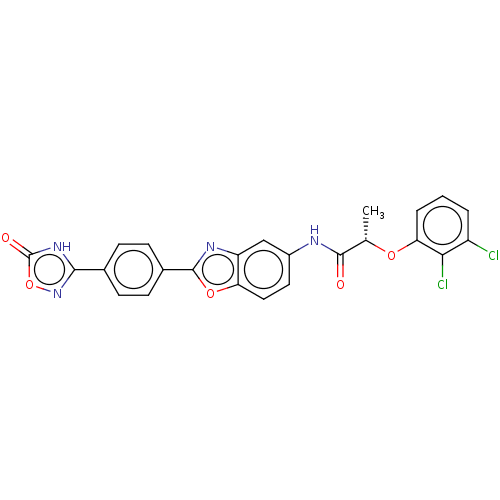 (CHEMBL4205894) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456073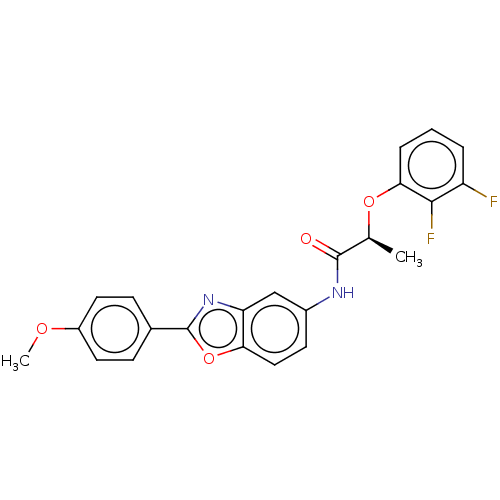 (CHEMBL4204321) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456075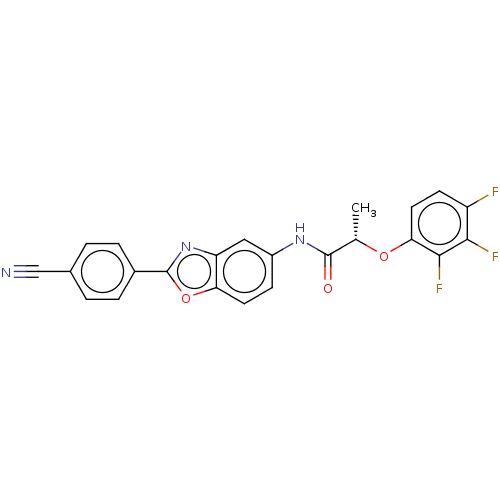 (CHEMBL4216354) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456102 (CHEMBL4207404) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456081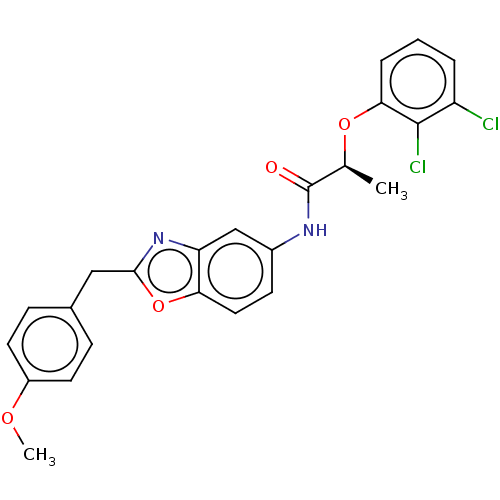 (CHEMBL4210836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50432765 (CHEMBL2348794) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456106 (CHEMBL4207207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 835 total ) | Next | Last >> |