 Found 402 hits with Last Name = 'feyen' and Initial = 'jh'
Found 402 hits with Last Name = 'feyen' and Initial = 'jh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256265
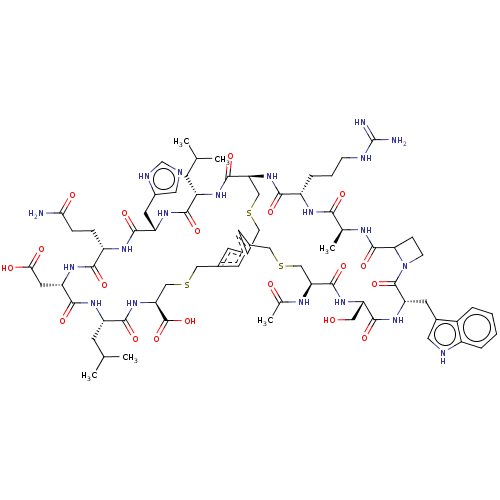
(CHEMBL4089486)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N1CCC1C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C74H104N20O19S3/c1-36(2)18-49-64(103)88-51(24-44-27-78-35-81-44)66(105)85-48(13-14-59(75)97)63(102)89-52(25-60(98)99)67(106)86-50(19-37(3)4)65(104)93-57(73(112)113)34-116-31-42-21-40-20-41(22-42)30-115-33-56(70(109)87-49)92-62(101)47(12-9-16-79-74(76)77)84-61(100)38(5)82-71(110)58-15-17-94(58)72(111)53(23-43-26-80-46-11-8-7-10-45(43)46)90-68(107)54(28-95)91-69(108)55(32-114-29-40)83-39(6)96/h7-8,10-11,20-22,26-27,35-38,47-58,80,95H,9,12-19,23-25,28-34H2,1-6H3,(H2,75,97)(H,78,81)(H,82,110)(H,83,96)(H,84,100)(H,85,105)(H,86,106)(H,87,109)(H,88,103)(H,89,102)(H,90,107)(H,91,108)(H,92,101)(H,93,104)(H,98,99)(H,112,113)(H4,76,77,79)/t38-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256283
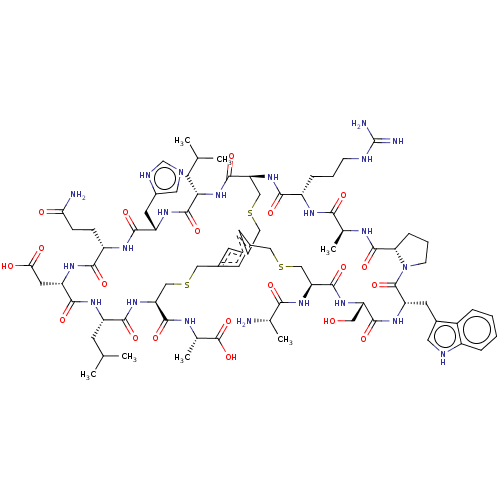
(CHEMBL4079711)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(O)=O)cc(CSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C79H114N22O20S3/c1-38(2)20-52-68(110)94-54(26-47-29-84-37-87-47)70(112)91-51(16-17-62(81)103)67(109)95-55(27-63(104)105)71(113)92-53(21-39(3)4)69(111)100-58(73(115)89-42(7)78(120)121)34-122-31-43-22-44-24-45(23-43)33-124-36-60(74(116)93-52)99-66(108)50(14-10-18-85-79(82)83)90-65(107)41(6)88-76(118)61-15-11-19-101(61)77(119)56(25-46-28-86-49-13-9-8-12-48(46)49)96-72(114)57(30-102)97-75(117)59(35-123-32-44)98-64(106)40(5)80/h8-9,12-13,22-24,28-29,37-42,50-61,86,102H,10-11,14-21,25-27,30-36,80H2,1-7H3,(H2,81,103)(H,84,87)(H,88,118)(H,89,115)(H,90,107)(H,91,112)(H,92,113)(H,93,116)(H,94,110)(H,95,109)(H,96,114)(H,97,117)(H,98,106)(H,99,108)(H,100,111)(H,104,105)(H,120,121)(H4,82,83,85)/t40-,41-,42-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256264

(CHEMBL4099333)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N1CCC1C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C75H106N20O19S3/c1-37(2)19-50-65(104)89-52(25-45-28-79-36-82-45)67(106)86-49(14-15-60(76)98)64(103)90-53(26-61(99)100)68(107)87-51(20-38(3)4)66(105)94-58(74(113)114)35-117-32-43-22-41-21-42(23-43)31-116-34-57(71(110)88-50)93-63(102)48(13-9-10-17-80-75(77)78)85-62(101)39(5)83-72(111)59-16-18-95(59)73(112)54(24-44-27-81-47-12-8-7-11-46(44)47)91-69(108)55(29-96)92-70(109)56(33-115-30-41)84-40(6)97/h7-8,11-12,21-23,27-28,36-39,48-59,81,96H,9-10,13-20,24-26,29-35H2,1-6H3,(H2,76,98)(H,79,82)(H,83,111)(H,84,97)(H,85,101)(H,86,106)(H,87,107)(H,88,110)(H,89,104)(H,90,103)(H,91,108)(H,92,109)(H,93,102)(H,94,105)(H,99,100)(H,113,114)(H4,77,78,80)/t39-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256277

(CHEMBL4093698)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C75H106N20O19S3/c1-37(2)19-50-65(104)89-52(25-45-28-79-36-82-45)67(106)86-49(15-16-60(76)98)64(103)90-53(26-61(99)100)68(107)87-51(20-38(3)4)66(105)94-58(74(113)114)35-117-32-43-22-41-21-42(23-43)31-116-34-57(71(110)88-50)93-63(102)48(13-9-17-80-75(77)78)85-62(101)39(5)83-72(111)59-14-10-18-95(59)73(112)54(24-44-27-81-47-12-8-7-11-46(44)47)91-69(108)55(29-96)92-70(109)56(33-115-30-41)84-40(6)97/h7-8,11-12,21-23,27-28,36-39,48-59,81,96H,9-10,13-20,24-26,29-35H2,1-6H3,(H2,76,98)(H,79,82)(H,83,111)(H,84,97)(H,85,101)(H,86,106)(H,87,107)(H,88,110)(H,89,104)(H,90,103)(H,91,108)(H,92,109)(H,93,102)(H,94,105)(H,99,100)(H,113,114)(H4,77,78,80)/t39-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256286

(CHEMBL4076199)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C79H109N19O20S3/c1-41(2)23-53-68(107)91-56(30-49-32-83-40-85-49)71(110)88-52(18-19-64(80)102)67(106)92-57(31-65(103)104)72(111)89-54(24-42(3)4)69(108)97-62(78(117)118)39-121-36-48-26-46-25-47(27-48)35-120-38-61(75(114)90-53)96-66(105)51(13-9-10-21-84-79(81)82)87-70(109)55(28-45-14-16-50(101)17-15-45)93-76(115)63-20-22-98(63)77(116)58(29-44-11-7-6-8-12-44)94-73(112)59(33-99)95-74(113)60(37-119-34-46)86-43(5)100/h6-8,11-12,14-17,25-27,32,40-42,51-63,99,101H,9-10,13,18-24,28-31,33-39H2,1-5H3,(H2,80,102)(H,83,85)(H,86,100)(H,87,109)(H,88,110)(H,89,111)(H,90,114)(H,91,107)(H,92,106)(H,93,115)(H,94,112)(H,95,113)(H,96,105)(H,97,108)(H,103,104)(H,117,118)(H4,81,82,84)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256260

(CHEMBL4094403)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC[C@H](C)NC(=O)[C@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H105N19O19S3/c1-40(2)22-53-67(105)94-60(75(113)114)38-117-35-47-24-45-23-46(25-47)34-116-37-59(71(109)84-41(3)30-82-52(28-48-31-80-39-83-48)66(104)87-51(17-18-62(77)99)65(103)89-55(29-63(100)101)69(107)88-53)93-64(102)50(12-8-9-20-81-76(78)79)86-68(106)54(26-44-13-15-49(98)16-14-44)90-73(111)61-19-21-95(61)74(112)56(27-43-10-6-5-7-11-43)91-70(108)57(32-96)92-72(110)58(36-115-33-45)85-42(4)97/h5-7,10-11,13-16,23-25,31,39-41,50-61,82,96,98H,8-9,12,17-22,26-30,32-38H2,1-4H3,(H2,77,99)(H,80,83)(H,84,109)(H,85,97)(H,86,106)(H,87,104)(H,88,107)(H,89,103)(H,90,111)(H,91,108)(H,92,110)(H,93,102)(H,94,105)(H,100,101)(H,113,114)(H4,78,79,81)/t41-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59+,60-,61?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256276
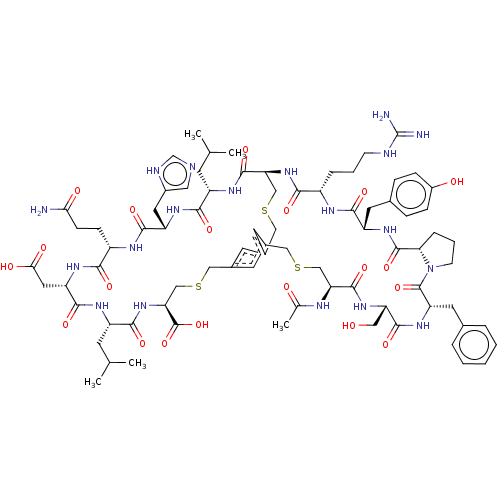
(CHEMBL4079260)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C79H109N19O20S3/c1-41(2)23-53-68(107)91-56(30-49-32-83-40-85-49)71(110)88-52(19-20-64(80)102)67(106)92-57(31-65(103)104)72(111)89-54(24-42(3)4)69(108)97-62(78(117)118)39-121-36-48-26-46-25-47(27-48)35-120-38-61(75(114)90-53)96-66(105)51(13-9-21-84-79(81)82)87-70(109)55(28-45-15-17-50(101)18-16-45)93-76(115)63-14-10-22-98(63)77(116)58(29-44-11-7-6-8-12-44)94-73(112)59(33-99)95-74(113)60(37-119-34-46)86-43(5)100/h6-8,11-12,15-18,25-27,32,40-42,51-63,99,101H,9-10,13-14,19-24,28-31,33-39H2,1-5H3,(H2,80,102)(H,83,85)(H,86,100)(H,87,109)(H,88,110)(H,89,111)(H,90,114)(H,91,107)(H,92,106)(H,93,115)(H,94,112)(H,95,113)(H,96,105)(H,97,108)(H,103,104)(H,117,118)(H4,81,82,84)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Rattus norvegicus) | BDBM50256284
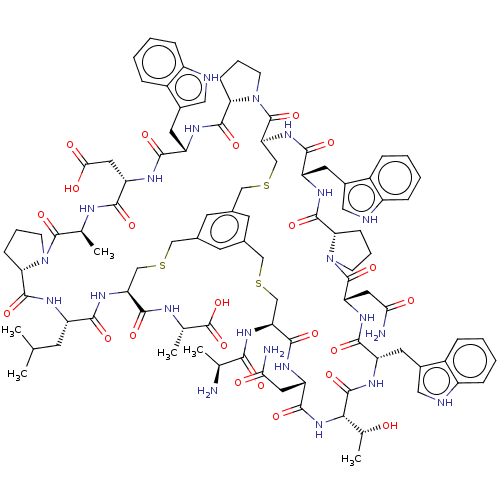
(CHEMBL4075544)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(O)=O)cc(CSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C97H124N22O23S3/c1-47(2)28-64-83(127)114-71(88(132)105-50(5)97(141)142)44-143-41-52-29-53-31-54(30-52)43-145-46-73(96(140)119-27-15-24-76(119)92(136)109-65(32-55-38-101-61-19-10-7-16-58(55)61)84(128)107-69(37-79(123)124)82(126)104-49(4)94(138)117-25-13-22-74(117)90(134)108-64)115-86(130)66(33-56-39-102-62-20-11-8-17-59(56)62)110-91(135)75-23-14-26-118(75)95(139)70(36-78(100)122)112-85(129)67(34-57-40-103-63-21-12-9-18-60(57)63)111-93(137)80(51(6)120)116-87(131)68(35-77(99)121)106-89(133)72(45-144-42-53)113-81(125)48(3)98/h7-12,16-21,29-31,38-40,47-51,64-76,80,101-103,120H,13-15,22-28,32-37,41-46,98H2,1-6H3,(H2,99,121)(H2,100,122)(H,104,126)(H,105,132)(H,106,133)(H,107,128)(H,108,134)(H,109,136)(H,110,135)(H,111,137)(H,112,129)(H,113,125)(H,114,127)(H,115,130)(H,116,131)(H,123,124)(H,141,142)/t48-,49-,50-,51+,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,80-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256288
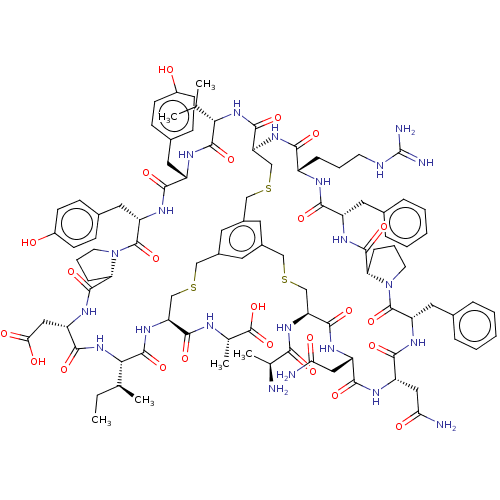
(CHEMBL4085408)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc4ccccc4)C(=O)N4CCC[C@H]4C(=O)N[C@@H](Cc4ccccc4)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(O)=O)c3)C(C)C |r| Show InChI InChI=1S/C99H132N22O24S3/c1-7-52(4)81-95(141)117-72(89(135)106-54(6)98(144)145)48-146-45-59-35-60-37-61(36-59)47-148-50-74(91(137)118-80(51(2)3)94(140)112-66(39-57-24-28-62(122)29-25-57)85(131)113-71(41-58-26-30-63(123)31-27-58)97(143)121-34-16-23-76(121)93(139)111-69(44-79(126)127)88(134)119-81)116-83(129)64(21-14-32-105-99(103)104)107-84(130)65(38-55-17-10-8-11-18-55)110-92(138)75-22-15-33-120(75)96(142)70(40-56-19-12-9-13-20-56)114-87(133)68(43-78(102)125)108-86(132)67(42-77(101)124)109-90(136)73(49-147-46-60)115-82(128)53(5)100/h8-13,17-20,24-31,35-37,51-54,64-76,80-81,122-123H,7,14-16,21-23,32-34,38-50,100H2,1-6H3,(H2,101,124)(H2,102,125)(H,106,135)(H,107,130)(H,108,132)(H,109,136)(H,110,138)(H,111,139)(H,112,140)(H,113,131)(H,114,133)(H,115,128)(H,116,129)(H,117,141)(H,118,137)(H,119,134)(H,126,127)(H,144,145)(H4,103,104,105)/t52-,53-,54-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,80-,81-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Rattus norvegicus) | BDBM50256283

(CHEMBL4079711)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(O)=O)cc(CSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C79H114N22O20S3/c1-38(2)20-52-68(110)94-54(26-47-29-84-37-87-47)70(112)91-51(16-17-62(81)103)67(109)95-55(27-63(104)105)71(113)92-53(21-39(3)4)69(111)100-58(73(115)89-42(7)78(120)121)34-122-31-43-22-44-24-45(23-43)33-124-36-60(74(116)93-52)99-66(108)50(14-10-18-85-79(82)83)90-65(107)41(6)88-76(118)61-15-11-19-101(61)77(119)56(25-46-28-86-49-13-9-8-12-48(46)49)96-72(114)57(30-102)97-75(117)59(35-123-32-44)98-64(106)40(5)80/h8-9,12-13,22-24,28-29,37-42,50-61,86,102H,10-11,14-21,25-27,30-36,80H2,1-7H3,(H2,81,103)(H,84,87)(H,88,118)(H,89,115)(H,90,107)(H,91,112)(H,92,113)(H,93,116)(H,94,110)(H,95,109)(H,96,114)(H,97,117)(H,98,106)(H,99,108)(H,100,111)(H,104,105)(H,120,121)(H4,82,83,85)/t40-,41-,42-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256263
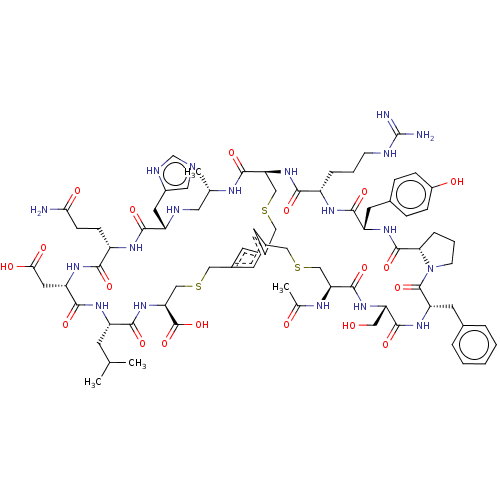
(CHEMBL4088185)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC[C@H](C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H105N19O19S3/c1-40(2)22-53-67(105)94-60(75(113)114)38-117-35-47-24-45-23-46(25-47)34-116-37-59(71(109)84-41(3)30-82-52(28-48-31-80-39-83-48)66(104)87-51(18-19-62(77)99)65(103)89-55(29-63(100)101)69(107)88-53)93-64(102)50(12-8-20-81-76(78)79)86-68(106)54(26-44-14-16-49(98)17-15-44)90-73(111)61-13-9-21-95(61)74(112)56(27-43-10-6-5-7-11-43)91-70(108)57(32-96)92-72(110)58(36-115-33-45)85-42(4)97/h5-7,10-11,14-17,23-25,31,39-41,50-61,82,96,98H,8-9,12-13,18-22,26-30,32-38H2,1-4H3,(H2,77,99)(H,80,83)(H,84,109)(H,85,97)(H,86,106)(H,87,104)(H,88,107)(H,89,103)(H,90,111)(H,91,108)(H,92,110)(H,93,102)(H,94,105)(H,100,101)(H,113,114)(H4,78,79,81)/t41-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Rattus norvegicus) | BDBM50256277

(CHEMBL4093698)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C75H106N20O19S3/c1-37(2)19-50-65(104)89-52(25-45-28-79-36-82-45)67(106)86-49(15-16-60(76)98)64(103)90-53(26-61(99)100)68(107)87-51(20-38(3)4)66(105)94-58(74(113)114)35-117-32-43-22-41-21-42(23-43)31-116-34-57(71(110)88-50)93-63(102)48(13-9-17-80-75(77)78)85-62(101)39(5)83-72(111)59-14-10-18-95(59)73(112)54(24-44-27-81-47-12-8-7-11-46(44)47)91-69(108)55(29-96)92-70(109)56(33-115-30-41)84-40(6)97/h7-8,11-12,21-23,27-28,36-39,48-59,81,96H,9-10,13-20,24-26,29-35H2,1-6H3,(H2,76,98)(H,79,82)(H,83,111)(H,84,97)(H,85,101)(H,86,106)(H,87,107)(H,88,110)(H,89,104)(H,90,103)(H,91,108)(H,92,109)(H,93,102)(H,94,105)(H,99,100)(H,113,114)(H4,77,78,80)/t39-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256287
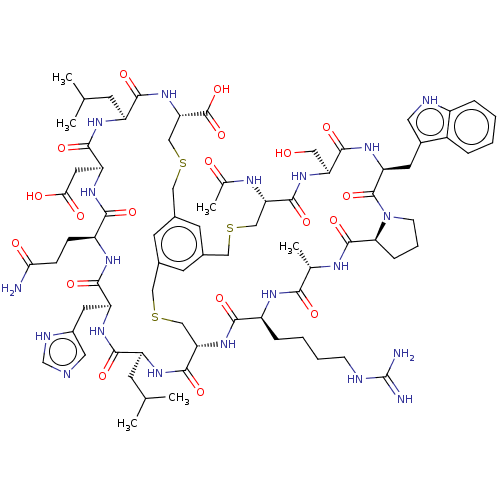
(CHEMBL4105318)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc4cnc[nH]4)NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H108N20O19S3/c1-38(2)20-51-66(105)90-53(26-46-29-80-37-83-46)68(107)87-50(16-17-61(77)99)65(104)91-54(27-62(100)101)69(108)88-52(21-39(3)4)67(106)95-59(75(114)115)36-118-33-44-23-42-22-43(24-44)32-117-35-58(72(111)89-51)94-64(103)49(14-9-10-18-81-76(78)79)86-63(102)40(5)84-73(112)60-15-11-19-96(60)74(113)55(25-45-28-82-48-13-8-7-12-47(45)48)92-70(109)56(30-97)93-71(110)57(34-116-31-42)85-41(6)98/h7-8,12-13,22-24,28-29,37-40,49-60,82,97H,9-11,14-21,25-27,30-36H2,1-6H3,(H2,77,99)(H,80,83)(H,84,112)(H,85,98)(H,86,102)(H,87,107)(H,88,108)(H,89,111)(H,90,105)(H,91,104)(H,92,109)(H,93,110)(H,94,103)(H,95,106)(H,100,101)(H,114,115)(H4,78,79,81)/t40-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256284

(CHEMBL4075544)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(O)=O)cc(CSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C97H124N22O23S3/c1-47(2)28-64-83(127)114-71(88(132)105-50(5)97(141)142)44-143-41-52-29-53-31-54(30-52)43-145-46-73(96(140)119-27-15-24-76(119)92(136)109-65(32-55-38-101-61-19-10-7-16-58(55)61)84(128)107-69(37-79(123)124)82(126)104-49(4)94(138)117-25-13-22-74(117)90(134)108-64)115-86(130)66(33-56-39-102-62-20-11-8-17-59(56)62)110-91(135)75-23-14-26-118(75)95(139)70(36-78(100)122)112-85(129)67(34-57-40-103-63-21-12-9-18-60(57)63)111-93(137)80(51(6)120)116-87(131)68(35-77(99)121)106-89(133)72(45-144-42-53)113-81(125)48(3)98/h7-12,16-21,29-31,38-40,47-51,64-76,80,101-103,120H,13-15,22-28,32-37,41-46,98H2,1-6H3,(H2,99,121)(H2,100,122)(H,104,126)(H,105,132)(H,106,133)(H,107,128)(H,108,134)(H,109,136)(H,110,135)(H,111,137)(H,112,129)(H,113,125)(H,114,127)(H,115,130)(H,116,131)(H,123,124)(H,141,142)/t48-,49-,50-,51+,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,80-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Rattus norvegicus) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein
(Sus scrofa) | BDBM50256277

(CHEMBL4093698)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C75H106N20O19S3/c1-37(2)19-50-65(104)89-52(25-45-28-79-36-82-45)67(106)86-49(15-16-60(76)98)64(103)90-53(26-61(99)100)68(107)87-51(20-38(3)4)66(105)94-58(74(113)114)35-117-32-43-22-41-21-42(23-43)31-116-34-57(71(110)88-50)93-63(102)48(13-9-17-80-75(77)78)85-62(101)39(5)83-72(111)59-14-10-18-95(59)73(112)54(24-44-27-81-47-12-8-7-11-46(44)47)91-69(108)55(29-96)92-70(109)56(33-115-30-41)84-40(6)97/h7-8,11-12,21-23,27-28,36-39,48-59,81,96H,9-10,13-20,24-26,29-35H2,1-6H3,(H2,76,98)(H,79,82)(H,83,111)(H,84,97)(H,85,101)(H,86,106)(H,87,107)(H,88,110)(H,89,104)(H,90,103)(H,91,108)(H,92,109)(H,93,102)(H,94,105)(H,99,100)(H,113,114)(H4,77,78,80)/t39-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-/m0/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of pig plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate addition ... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Rattus norvegicus) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Rattus norvegicus) | BDBM50256276

(CHEMBL4079260)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C79H109N19O20S3/c1-41(2)23-53-68(107)91-56(30-49-32-83-40-85-49)71(110)88-52(19-20-64(80)102)67(106)92-57(31-65(103)104)72(111)89-54(24-42(3)4)69(108)97-62(78(117)118)39-121-36-48-26-46-25-47(27-48)35-120-38-61(75(114)90-53)96-66(105)51(13-9-21-84-79(81)82)87-70(109)55(28-45-15-17-50(101)18-16-45)93-76(115)63-14-10-22-98(63)77(116)58(29-44-11-7-6-8-12-44)94-73(112)59(33-99)95-74(113)60(37-119-34-46)86-43(5)100/h6-8,11-12,15-18,25-27,32,40-42,51-63,99,101H,9-10,13-14,19-24,28-31,33-39H2,1-5H3,(H2,80,102)(H,83,85)(H,86,100)(H,87,109)(H,88,110)(H,89,111)(H,90,114)(H,91,107)(H,92,106)(H,93,115)(H,94,112)(H,95,113)(H,96,105)(H,97,108)(H,103,104)(H,117,118)(H4,81,82,84)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein
(Sus scrofa) | BDBM50256276

(CHEMBL4079260)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C79H109N19O20S3/c1-41(2)23-53-68(107)91-56(30-49-32-83-40-85-49)71(110)88-52(19-20-64(80)102)67(106)92-57(31-65(103)104)72(111)89-54(24-42(3)4)69(108)97-62(78(117)118)39-121-36-48-26-46-25-47(27-48)35-120-38-61(75(114)90-53)96-66(105)51(13-9-21-84-79(81)82)87-70(109)55(28-45-15-17-50(101)18-16-45)93-76(115)63-14-10-22-98(63)77(116)58(29-44-11-7-6-8-12-44)94-73(112)59(33-99)95-74(113)60(37-119-34-46)86-43(5)100/h6-8,11-12,15-18,25-27,32,40-42,51-63,99,101H,9-10,13-14,19-24,28-31,33-39H2,1-5H3,(H2,80,102)(H,83,85)(H,86,100)(H,87,109)(H,88,110)(H,89,111)(H,90,114)(H,91,107)(H,92,106)(H,93,115)(H,94,112)(H,95,113)(H,96,105)(H,97,108)(H,103,104)(H,117,118)(H4,81,82,84)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of pig plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate addition ... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Rattus norvegicus) | BDBM50256260

(CHEMBL4094403)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC[C@H](C)NC(=O)[C@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H105N19O19S3/c1-40(2)22-53-67(105)94-60(75(113)114)38-117-35-47-24-45-23-46(25-47)34-116-37-59(71(109)84-41(3)30-82-52(28-48-31-80-39-83-48)66(104)87-51(17-18-62(77)99)65(103)89-55(29-63(100)101)69(107)88-53)93-64(102)50(12-8-9-20-81-76(78)79)86-68(106)54(26-44-13-15-49(98)16-14-44)90-73(111)61-19-21-95(61)74(112)56(27-43-10-6-5-7-11-43)91-70(108)57(32-96)92-72(110)58(36-115-33-45)85-42(4)97/h5-7,10-11,13-16,23-25,31,39-41,50-61,82,96,98H,8-9,12,17-22,26-30,32-38H2,1-4H3,(H2,77,99)(H,80,83)(H,84,109)(H,85,97)(H,86,106)(H,87,104)(H,88,107)(H,89,103)(H,90,111)(H,91,108)(H,92,110)(H,93,102)(H,94,105)(H,100,101)(H,113,114)(H4,78,79,81)/t41-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59+,60-,61?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Mus musculus) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse C-terminal 6His-tagged plasma kallikrein (20 to 638 residues) using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrat... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Mus musculus) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse C-terminal 6His-tagged plasma kallikrein (20 to 638 residues) using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrat... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Mus musculus) | BDBM50256260

(CHEMBL4094403)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC[C@H](C)NC(=O)[C@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H105N19O19S3/c1-40(2)22-53-67(105)94-60(75(113)114)38-117-35-47-24-45-23-46(25-47)34-116-37-59(71(109)84-41(3)30-82-52(28-48-31-80-39-83-48)66(104)87-51(17-18-62(77)99)65(103)89-55(29-63(100)101)69(107)88-53)93-64(102)50(12-8-9-20-81-76(78)79)86-68(106)54(26-44-13-15-49(98)16-14-44)90-73(111)61-19-21-95(61)74(112)56(27-43-10-6-5-7-11-43)91-70(108)57(32-96)92-72(110)58(36-115-33-45)85-42(4)97/h5-7,10-11,13-16,23-25,31,39-41,50-61,82,96,98H,8-9,12,17-22,26-30,32-38H2,1-4H3,(H2,77,99)(H,80,83)(H,84,109)(H,85,97)(H,86,106)(H,87,104)(H,88,107)(H,89,103)(H,90,111)(H,91,108)(H,92,110)(H,93,102)(H,94,105)(H,100,101)(H,113,114)(H4,78,79,81)/t41-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59+,60-,61?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse C-terminal 6His-tagged plasma kallikrein (20 to 638 residues) using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrat... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein
(Sus scrofa) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein
(Sus scrofa) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of pig plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate addition ... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using fluorogenic peptide as substrate by fluorescence based assay |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein
(Sus scrofa) | BDBM50256260

(CHEMBL4094403)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC[C@H](C)NC(=O)[C@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H105N19O19S3/c1-40(2)22-53-67(105)94-60(75(113)114)38-117-35-47-24-45-23-46(25-47)34-116-37-59(71(109)84-41(3)30-82-52(28-48-31-80-39-83-48)66(104)87-51(17-18-62(77)99)65(103)89-55(29-63(100)101)69(107)88-53)93-64(102)50(12-8-9-20-81-76(78)79)86-68(106)54(26-44-13-15-49(98)16-14-44)90-73(111)61-19-21-95(61)74(112)56(27-43-10-6-5-7-11-43)91-70(108)57(32-96)92-72(110)58(36-115-33-45)85-42(4)97/h5-7,10-11,13-16,23-25,31,39-41,50-61,82,96,98H,8-9,12,17-22,26-30,32-38H2,1-4H3,(H2,77,99)(H,80,83)(H,84,109)(H,85,97)(H,86,106)(H,87,104)(H,88,107)(H,89,103)(H,90,111)(H,91,108)(H,92,110)(H,93,102)(H,94,105)(H,100,101)(H,113,114)(H4,78,79,81)/t41-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59+,60-,61?/m0/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of pig plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate addition ... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein-5
(Homo sapiens (Human)) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal 10His-tagged KLK5 (67 to 293 residues) expressed in mouse NS0 cells using fluorogenic Boc-VPR-AMC peptide ... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein-13
(Homo sapiens) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50256285
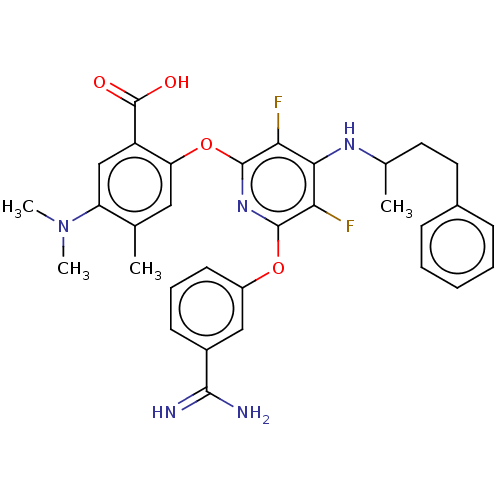
(CHEMBL4086746)Show SMILES CC(CCc1ccccc1)Nc1c(F)c(Oc2cccc(c2)C(N)=N)nc(Oc2cc(C)c(cc2C(O)=O)N(C)C)c1F Show InChI InChI=1S/C32H33F2N5O4/c1-18-15-25(23(32(40)41)17-24(18)39(3)4)43-31-27(34)28(37-19(2)13-14-20-9-6-5-7-10-20)26(33)30(38-31)42-22-12-8-11-21(16-22)29(35)36/h5-12,15-17,19H,13-14H2,1-4H3,(H3,35,36)(H,37,38)(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of factor 11a (unknown origin) |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein-14
(Homo sapiens (Human)) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal 10His-tagged KLK14 (19 to 248 residues) expressed in mouse NS0 cells using fluorogenic Boc-VPR-AMC peptide... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein-2
(Homo sapiens (Human)) | BDBM50256260

(CHEMBL4094403)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC[C@H](C)NC(=O)[C@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H105N19O19S3/c1-40(2)22-53-67(105)94-60(75(113)114)38-117-35-47-24-45-23-46(25-47)34-116-37-59(71(109)84-41(3)30-82-52(28-48-31-80-39-83-48)66(104)87-51(17-18-62(77)99)65(103)89-55(29-63(100)101)69(107)88-53)93-64(102)50(12-8-9-20-81-76(78)79)86-68(106)54(26-44-13-15-49(98)16-14-44)90-73(111)61-19-21-95(61)74(112)56(27-43-10-6-5-7-11-43)91-70(108)57(32-96)92-72(110)58(36-115-33-45)85-42(4)97/h5-7,10-11,13-16,23-25,31,39-41,50-61,82,96,98H,8-9,12,17-22,26-30,32-38H2,1-4H3,(H2,77,99)(H,80,83)(H,84,109)(H,85,97)(H,86,106)(H,87,104)(H,88,107)(H,89,103)(H,90,111)(H,91,108)(H,92,110)(H,93,102)(H,94,105)(H,100,101)(H,113,114)(H4,78,79,81)/t41-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59+,60-,61?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal 10His-tagged KLK2 (1 to 261 residues) expressed in mouse NS0 cells using fluorogenic PFR-AMC peptide as su... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein-2
(Homo sapiens (Human)) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal 10His-tagged KLK2 (1 to 261 residues) expressed in mouse NS0 cells using fluorogenic PFR-AMC peptide as su... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal 6His-tagged matriptase catalytic domain (596 to 855 residues) expressed in Escherichia coli using fluoroge... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal 10His-tagged factor Xa (24 to 488 residues) expressed in baculovirus derived insect sf21 cells using fluor... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal 6His-tagged matriptase catalytic domain (596 to 855 residues) expressed in Escherichia coli using fluoroge... |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Kallikrein-12
(Homo sapiens) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50256260

(CHEMBL4094403)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC[C@H](C)NC(=O)[C@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H105N19O19S3/c1-40(2)22-53-67(105)94-60(75(113)114)38-117-35-47-24-45-23-46(25-47)34-116-37-59(71(109)84-41(3)30-82-52(28-48-31-80-39-83-48)66(104)87-51(17-18-62(77)99)65(103)89-55(29-63(100)101)69(107)88-53)93-64(102)50(12-8-9-20-81-76(78)79)86-68(106)54(26-44-13-15-49(98)16-14-44)90-73(111)61-19-21-95(61)74(112)56(27-43-10-6-5-7-11-43)91-70(108)57(32-96)92-72(110)58(36-115-33-45)85-42(4)97/h5-7,10-11,13-16,23-25,31,39-41,50-61,82,96,98H,8-9,12,17-22,26-30,32-38H2,1-4H3,(H2,77,99)(H,80,83)(H,84,109)(H,85,97)(H,86,106)(H,87,104)(H,88,107)(H,89,103)(H,90,111)(H,91,108)(H,92,110)(H,93,102)(H,94,105)(H,100,101)(H,113,114)(H4,78,79,81)/t41-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59+,60-,61?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50256260

(CHEMBL4094403)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC[C@H](C)NC(=O)[C@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H105N19O19S3/c1-40(2)22-53-67(105)94-60(75(113)114)38-117-35-47-24-45-23-46(25-47)34-116-37-59(71(109)84-41(3)30-82-52(28-48-31-80-39-83-48)66(104)87-51(17-18-62(77)99)65(103)89-55(29-63(100)101)69(107)88-53)93-64(102)50(12-8-9-20-81-76(78)79)86-68(106)54(26-44-13-15-49(98)16-14-44)90-73(111)61-19-21-95(61)74(112)56(27-43-10-6-5-7-11-43)91-70(108)57(32-96)92-72(110)58(36-115-33-45)85-42(4)97/h5-7,10-11,13-16,23-25,31,39-41,50-61,82,96,98H,8-9,12,17-22,26-30,32-38H2,1-4H3,(H2,77,99)(H,80,83)(H,84,109)(H,85,97)(H,86,106)(H,87,104)(H,88,107)(H,89,103)(H,90,111)(H,91,108)(H,92,110)(H,93,102)(H,94,105)(H,100,101)(H,113,114)(H4,78,79,81)/t41-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59+,60-,61?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of chimeric PDGF receptor with FLT-3 cytoplasmic domain phosphorylation in CHO cells |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM50256262

(CHEMBL4070056)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSCc3cc(CSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3)C(C)C |r| Show InChI InChI=1S/C114H161N29O28S3/c1-10-62(4)94-107(166)136-85(101(160)124-63(5)108(167)141(9)53-91(151)140(8)52-90(150)139(7)51-89(149)126-75(22-16-39-122-113(117)118)95(154)128-77(111(170)171)23-17-40-123-114(119)120)59-173-56-70-43-69-44-71(45-70)57-174-60-86(103(162)137-93(61(2)3)106(165)131-79(47-67-27-33-73(147)34-28-67)98(157)132-82(49-68-29-35-74(148)36-30-68)109(168)142-41-18-24-87(142)104(163)130-80(50-92(152)153)99(158)138-94)135-96(155)76(21-14-15-38-121-112(115)116)127-97(156)78(46-66-25-31-72(146)32-26-66)129-105(164)88-37-42-143(88)110(169)81(48-65-19-12-11-13-20-65)133-100(159)83(54-144)134-102(161)84(58-172-55-69)125-64(6)145/h11-13,19-20,25-36,43-45,61-63,75-88,93-94,144,146-148H,10,14-18,21-24,37-42,46-60H2,1-9H3,(H,124,160)(H,125,145)(H,126,149)(H,127,156)(H,128,154)(H,129,164)(H,130,163)(H,131,165)(H,132,157)(H,133,159)(H,134,161)(H,135,155)(H,136,166)(H,137,162)(H,138,158)(H,152,153)(H,170,171)(H4,115,116,121)(H4,117,118,122)(H4,119,120,123)/t62-,63-,75+,76-,77+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88?,93-,94-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50256260

(CHEMBL4094403)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC[C@H](C)NC(=O)[C@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H105N19O19S3/c1-40(2)22-53-67(105)94-60(75(113)114)38-117-35-47-24-45-23-46(25-47)34-116-37-59(71(109)84-41(3)30-82-52(28-48-31-80-39-83-48)66(104)87-51(17-18-62(77)99)65(103)89-55(29-63(100)101)69(107)88-53)93-64(102)50(12-8-9-20-81-76(78)79)86-68(106)54(26-44-13-15-49(98)16-14-44)90-73(111)61-19-21-95(61)74(112)56(27-43-10-6-5-7-11-43)91-70(108)57(32-96)92-72(110)58(36-115-33-45)85-42(4)97/h5-7,10-11,13-16,23-25,31,39-41,50-61,82,96,98H,8-9,12,17-22,26-30,32-38H2,1-4H3,(H2,77,99)(H,80,83)(H,84,109)(H,85,97)(H,86,106)(H,87,104)(H,88,107)(H,89,103)(H,90,111)(H,91,108)(H,92,110)(H,93,102)(H,94,105)(H,100,101)(H,113,114)(H4,78,79,81)/t41-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59+,60-,61?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50256260

(CHEMBL4094403)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cnc[nH]2)NC[C@H](C)NC(=O)[C@H]2CSCc3cc(CSC[C@H](NC1=O)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N2)c3 |r| Show InChI InChI=1S/C76H105N19O19S3/c1-40(2)22-53-67(105)94-60(75(113)114)38-117-35-47-24-45-23-46(25-47)34-116-37-59(71(109)84-41(3)30-82-52(28-48-31-80-39-83-48)66(104)87-51(17-18-62(77)99)65(103)89-55(29-63(100)101)69(107)88-53)93-64(102)50(12-8-9-20-81-76(78)79)86-68(106)54(26-44-13-15-49(98)16-14-44)90-73(111)61-19-21-95(61)74(112)56(27-43-10-6-5-7-11-43)91-70(108)57(32-96)92-72(110)58(36-115-33-45)85-42(4)97/h5-7,10-11,13-16,23-25,31,39-41,50-61,82,96,98H,8-9,12,17-22,26-30,32-38H2,1-4H3,(H2,77,99)(H,80,83)(H,84,109)(H,85,97)(H,86,106)(H,87,104)(H,88,107)(H,89,103)(H,90,111)(H,91,108)(H,92,110)(H,93,102)(H,94,105)(H,100,101)(H,113,114)(H4,78,79,81)/t41-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59+,60-,61?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50256261

(CHEMBL4059991)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CSCc3cc(CSC[C@@H](NC1=O)C(=O)N[C@@H](C)C(=O)N(C)CC(=O)N(C)CC(=O)N(C)CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)cc(CSC[C@H](NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N2)c3 |r| Show InChI InChI=1S/C117H160N32O28S3/c1-59(2)37-78-100(161)141-86(105(166)129-60(3)111(172)146(9)52-95(156)145(8)51-94(155)144(7)50-93(154)132-76(27-16-32-124-116(120)121)98(159)133-77(115(176)177)28-17-33-125-117(122)123)57-179-54-65-38-64-39-66(40-65)55-180-58-87(114(175)149-36-20-31-90(149)109(170)137-79(41-67-47-126-73-24-13-10-21-70(67)73)101(162)135-83(46-96(157)158)99(160)130-61(4)112(173)147-34-18-29-88(147)107(168)136-78)142-103(164)80(42-68-48-127-74-25-14-11-22-71(68)74)138-108(169)89-30-19-35-148(89)113(174)84(45-92(119)153)140-102(163)81(43-69-49-128-75-26-15-12-23-72(69)75)139-110(171)97(62(5)150)143-104(165)82(44-91(118)152)134-106(167)85(56-178-53-64)131-63(6)151/h10-15,21-26,38-40,47-49,59-62,76-90,97,126-128,150H,16-20,27-37,41-46,50-58H2,1-9H3,(H2,118,152)(H2,119,153)(H,129,166)(H,130,160)(H,131,151)(H,132,154)(H,133,159)(H,134,167)(H,135,162)(H,136,168)(H,137,170)(H,138,169)(H,139,171)(H,140,163)(H,141,161)(H,142,164)(H,143,165)(H,157,158)(H,176,177)(H4,120,121,124)(H4,122,123,125)/t60-,61-,62+,76+,77+,78-,79-,80-,81-,82-,83-,84-,85-,86+,87-,88-,89-,90-,97-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K.
Curated by ChEMBL
| Assay Description
Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma |
J Med Chem 61: 2823-2836 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01625
BindingDB Entry DOI: 10.7270/Q2862JW6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































