 Found 51 hits with Last Name = 'filipponi' and Initial = 'p'
Found 51 hits with Last Name = 'filipponi' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bile acid receptor
(Homo sapiens (Human)) | BDBM21674

((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as coactivator interaction with receptor ligand binding domain by Alphascreen assay |
Bioorg Med Chem 19: 2650-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.004
BindingDB Entry DOI: 10.7270/Q28W3DNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50354850

(BUDESONIDE | US10869929, Compound Budesonide | US1...)Show SMILES CCCC1O[C@@H]2C[C@H]3[C@@H]4CCC5=CC(=O)C=C[C@]5(C)[C@H]4[C@@H](O)C[C@]3(C)[C@@]2(O1)C(=O)CO |r,c:15,t:11| Show InChI InChI=1S/C25H34O6/c1-4-5-21-30-20-11-17-16-7-6-14-10-15(27)8-9-23(14,2)22(16)18(28)12-24(17,3)25(20,31-21)19(29)13-26/h8-10,16-18,20-22,26,28H,4-7,11-13H2,1-3H3/t16-,17-,18-,20+,21?,22+,23-,24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human GR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50190597
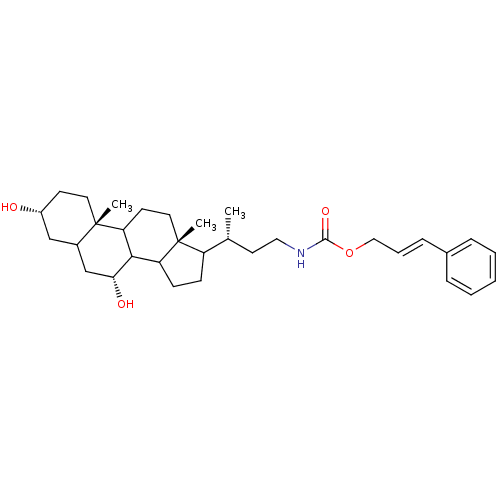
(23-N-(carbocinnamyloxy)-3alpha,7alpha-dihydroxy-24...)Show SMILES C[C@H](CCNC(=O)OC\C=C\c1ccccc1)C1CCC2C3[C@H](O)CC4C[C@H](O)CC[C@]4(C)C3CC[C@]12C Show InChI InChI=1S/C33H49NO4/c1-22(15-18-34-31(37)38-19-7-10-23-8-5-4-6-9-23)26-11-12-27-30-28(14-17-33(26,27)3)32(2)16-13-25(35)20-24(32)21-29(30)36/h4-10,22,24-30,35-36H,11-21H2,1-3H3,(H,34,37)/b10-7+/t22-,24?,25-,26?,27?,28?,29-,30?,32+,33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as coactivator interaction with receptor ligand binding domain by Alphascreen assay |
Bioorg Med Chem 19: 2650-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.004
BindingDB Entry DOI: 10.7270/Q28W3DNP |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50342396

(23-N-(carbocinnamyloxy)-3alpha,7alpha-dihydroxy-6a...)Show SMILES CC[C@H]1[C@@H](O)[C@H]2[C@@H]3CC[C@H]([C@H](C)CCNC(=O)OC\C=C\c4ccccc4)[C@@]3(C)CC[C@@H]2[C@@]2(C)CC[C@@H](O)C[C@@H]12 |r| Show InChI InChI=1S/C35H53NO4/c1-5-26-30-22-25(37)15-18-35(30,4)29-16-19-34(3)27(13-14-28(34)31(29)32(26)38)23(2)17-20-36-33(39)40-21-9-12-24-10-7-6-8-11-24/h6-12,23,25-32,37-38H,5,13-22H2,1-4H3,(H,36,39)/b12-9+/t23-,25-,26-,27-,28+,29+,30+,31+,32-,34-,35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as coactivator interaction with receptor ligand binding domain by Alphascreen assay |
Bioorg Med Chem 19: 2650-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.004
BindingDB Entry DOI: 10.7270/Q28W3DNP |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM21674

((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in NCI-H716 cells assessed as intracellular cAMP level by TR-FRET assay |
Bioorg Med Chem 19: 2650-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.004
BindingDB Entry DOI: 10.7270/Q28W3DNP |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM21675
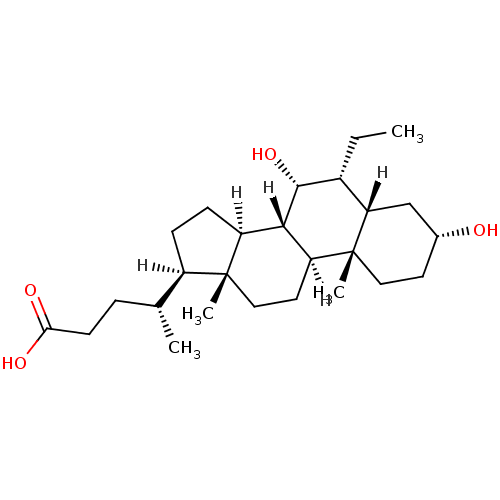
((4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-ethy...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in NCI-H716 cells assessed as intracellular cAMP level by TR-FRET assay |
Bioorg Med Chem 19: 2650-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.004
BindingDB Entry DOI: 10.7270/Q28W3DNP |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50190597

(23-N-(carbocinnamyloxy)-3alpha,7alpha-dihydroxy-24...)Show SMILES C[C@H](CCNC(=O)OC\C=C\c1ccccc1)C1CCC2C3[C@H](O)CC4C[C@H](O)CC[C@]4(C)C3CC[C@]12C Show InChI InChI=1S/C33H49NO4/c1-22(15-18-34-31(37)38-19-7-10-23-8-5-4-6-9-23)26-11-12-27-30-28(14-17-33(26,27)3)32(2)16-13-25(35)20-24(32)21-29(30)36/h4-10,22,24-30,35-36H,11-21H2,1-3H3,(H,34,37)/b10-7+/t22-,24?,25-,26?,27?,28?,29-,30?,32+,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in NCI-H716 cells assessed as intracellular cAMP level by TR-FRET assay |
Bioorg Med Chem 19: 2650-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.004
BindingDB Entry DOI: 10.7270/Q28W3DNP |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50342396

(23-N-(carbocinnamyloxy)-3alpha,7alpha-dihydroxy-6a...)Show SMILES CC[C@H]1[C@@H](O)[C@H]2[C@@H]3CC[C@H]([C@H](C)CCNC(=O)OC\C=C\c4ccccc4)[C@@]3(C)CC[C@@H]2[C@@]2(C)CC[C@@H](O)C[C@@H]12 |r| Show InChI InChI=1S/C35H53NO4/c1-5-26-30-22-25(37)15-18-35(30,4)29-16-19-34(3)27(13-14-28(34)31(29)32(26)38)23(2)17-20-36-33(39)40-21-9-12-24-10-7-6-8-11-24/h6-12,23,25-32,37-38H,5,13-22H2,1-4H3,(H,36,39)/b12-9+/t23-,25-,26-,27-,28+,29+,30+,31+,32-,34-,35-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in NCI-H716 cells assessed as intracellular cAMP level by TR-FRET assay |
Bioorg Med Chem 19: 2650-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.004
BindingDB Entry DOI: 10.7270/Q28W3DNP |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21674

((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at glutathione transferase-tagged human FXR-LBD using biotinylated Src-1 peptide incubated for 30 mins by recruitment coactivator as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM53721

((4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-10,13-dim...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20+,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at glutathione transferase-tagged human FXR-LBD using biotinylated Src-1 peptide incubated for 30 mins by recruitment coactivator as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21675

((4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-ethy...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at glutathione transferase-tagged human FXR-LBD using biotinylated Src-1 peptide incubated for 30 mins by recruitment coactivator as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50300199
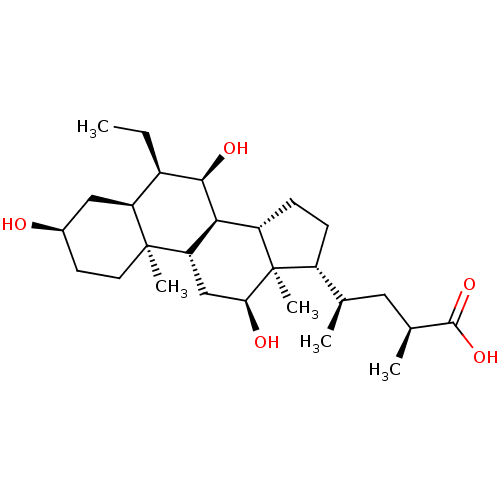
(6alpha-ethyl-23(S)-methyl-cholic acid | CHEMBL5676...)Show SMILES CC[C@H]1[C@@H](O)[C@H]2[C@@H]3CC[C@H]([C@H](C)C[C@H](C)C(O)=O)[C@@]3(C)[C@@H](O)C[C@@H]2[C@@]2(C)CC[C@@H](O)C[C@@H]12 |r| Show InChI InChI=1S/C27H46O5/c1-6-17-20-12-16(28)9-10-26(20,4)21-13-22(29)27(5)18(14(2)11-15(3)25(31)32)7-8-19(27)23(21)24(17)30/h14-24,28-30H,6-13H2,1-5H3,(H,31,32)/t14-,15+,16-,17-,18-,19+,20+,21+,22+,23+,24-,26+,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at glutathione transferase-tagged human FXR-LBD using biotinylated Src-1 peptide incubated for 30 mins by recruitment coactivator as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at glutathione transferase-tagged human FXR-LBD using biotinylated Src-1 peptide incubated for 30 mins by recruitment coactivator as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50550153

(CHEMBL4758363)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at glutathione transferase-tagged human FXR-LBD using biotinylated Src-1 peptide incubated for 30 mins by recruitment coactivator as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50550154

(CHEMBL4740844)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@@H](O)[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at glutathione transferase-tagged human FXR-LBD using biotinylated Src-1 peptide incubated for 30 mins by recruitment coactivator as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50300201
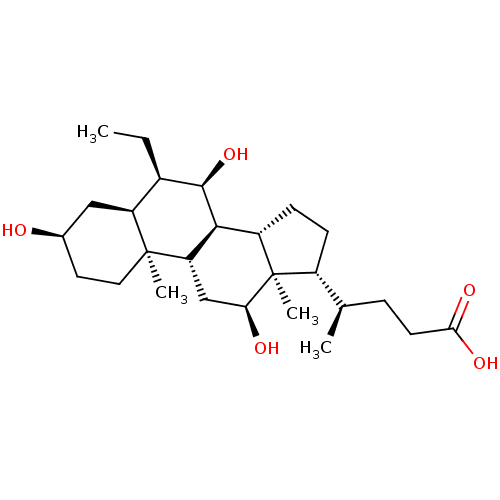
(3alpha,7alpha,12alpha-Trihydroxy-6alpha-ethyl-5bet...)Show SMILES CC[C@H]1[C@@H](O)[C@H]2[C@@H]3CC[C@H]([C@H](C)CCC(O)=O)[C@@]3(C)[C@@H](O)C[C@@H]2[C@@]2(C)CC[C@@H](O)C[C@@H]12 |r| Show InChI InChI=1S/C26H44O5/c1-5-16-19-12-15(27)10-11-25(19,3)20-13-21(28)26(4)17(14(2)6-9-22(29)30)7-8-18(26)23(20)24(16)31/h14-21,23-24,27-28,31H,5-13H2,1-4H3,(H,29,30)/t14-,15-,16-,17-,18+,19+,20+,21+,23+,24-,25+,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at glutathione transferase-tagged human FXR-LBD using biotinylated Src-1 peptide incubated for 30 mins by recruitment coactivator as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50550155
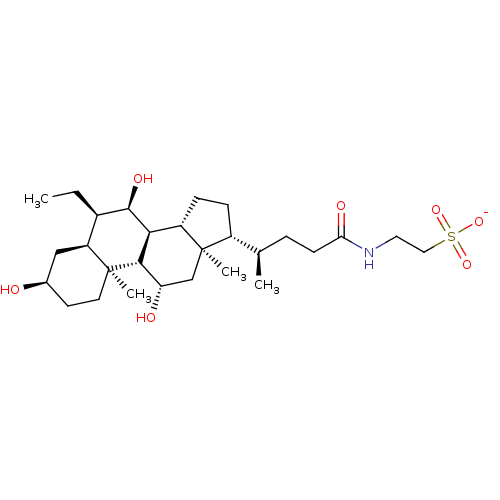
(CHEMBL4740500)Show SMILES [Na;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[C@]3([H])[#6@H](-[#8])-[#6@H](-[#6]-[#6])[C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6@@H](-[#8])-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]S([#8-])(=O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at glutathione transferase-tagged human FXR-LBD using biotinylated Src-1 peptide incubated for 30 mins by recruitment coactivator as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50550156

(CHEMBL4790262)Show SMILES [Na;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[C@]3([H])[#6@H](-[#8])-[#6@H](-[#6]-[#6])[C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6@@H](-[#8])-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6](-[#8-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at glutathione transferase-tagged human FXR-LBD using biotinylated Src-1 peptide incubated for 30 mins by recruitment coactivator as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM21674

((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TGR5 in human NCI-H716 cells assessed as stimulation of intracellular cAMP accumulation incubated for 60 mins by HTR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM53721

((4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-10,13-dim...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20+,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TGR5 in human NCI-H716 cells assessed as stimulation of intracellular cAMP accumulation incubated for 60 mins by HTR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM21675

((4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-ethy...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TGR5 in human NCI-H716 cells assessed as stimulation of intracellular cAMP accumulation incubated for 60 mins by HTR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50300199

(6alpha-ethyl-23(S)-methyl-cholic acid | CHEMBL5676...)Show SMILES CC[C@H]1[C@@H](O)[C@H]2[C@@H]3CC[C@H]([C@H](C)C[C@H](C)C(O)=O)[C@@]3(C)[C@@H](O)C[C@@H]2[C@@]2(C)CC[C@@H](O)C[C@@H]12 |r| Show InChI InChI=1S/C27H46O5/c1-6-17-20-12-16(28)9-10-26(20,4)21-13-22(29)27(5)18(14(2)11-15(3)25(31)32)7-8-19(27)23(21)24(17)30/h14-24,28-30H,6-13H2,1-5H3,(H,31,32)/t14-,15+,16-,17-,18-,19+,20+,21+,22+,23+,24-,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TGR5 in human NCI-H716 cells assessed as stimulation of intracellular cAMP accumulation incubated for 60 mins by HTR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TGR5 in human NCI-H716 cells assessed as stimulation of intracellular cAMP accumulation incubated for 60 mins by HTR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50550153

(CHEMBL4758363)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.15E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TGR5 in human NCI-H716 cells assessed as stimulation of intracellular cAMP accumulation incubated for 60 mins by HTR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50550154

(CHEMBL4740844)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@@H](O)[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TGR5 in human NCI-H716 cells assessed as stimulation of intracellular cAMP accumulation incubated for 60 mins by HTR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50300201

(3alpha,7alpha,12alpha-Trihydroxy-6alpha-ethyl-5bet...)Show SMILES CC[C@H]1[C@@H](O)[C@H]2[C@@H]3CC[C@H]([C@H](C)CCC(O)=O)[C@@]3(C)[C@@H](O)C[C@@H]2[C@@]2(C)CC[C@@H](O)C[C@@H]12 |r| Show InChI InChI=1S/C26H44O5/c1-5-16-19-12-15(27)10-11-25(19,3)20-13-21(28)26(4)17(14(2)6-9-22(29)30)7-8-18(26)23(20)24(16)31/h14-21,23-24,27-28,31H,5-13H2,1-4H3,(H,29,30)/t14-,15-,16-,17-,18+,19+,20+,21+,23+,24-,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TGR5 in human NCI-H716 cells assessed as stimulation of intracellular cAMP accumulation incubated for 60 mins by HTR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50550155

(CHEMBL4740500)Show SMILES [Na;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[C@]3([H])[#6@H](-[#8])-[#6@H](-[#6]-[#6])[C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6@@H](-[#8])-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]S([#8-])(=O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TGR5 in human NCI-H716 cells assessed as stimulation of intracellular cAMP accumulation incubated for 60 mins by HTR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50550156

(CHEMBL4790262)Show SMILES [Na;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[C@]3([H])[#6@H](-[#8])-[#6@H](-[#6]-[#6])[C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6@@H](-[#8])-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6](-[#8-])=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at TGR5 in human NCI-H716 cells assessed as stimulation of intracellular cAMP accumulation incubated for 60 mins by HTR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 3
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CAR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 3
(Homo sapiens (Human)) | BDBM50030476
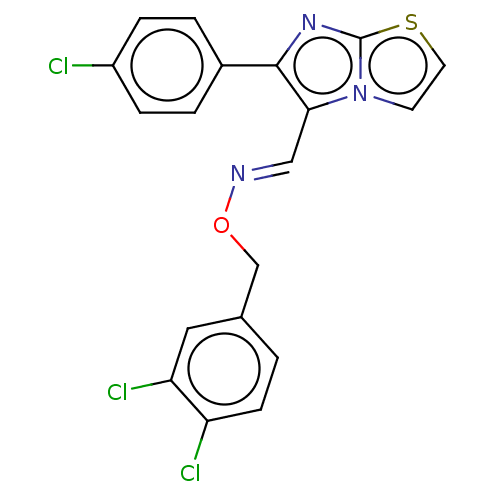
(CHEMBL458603)Show SMILES Clc1ccc(cc1)-c1nc2sccn2c1\C=N\OCc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H12Cl3N3OS/c20-14-4-2-13(3-5-14)18-17(25-7-8-27-19(25)24-18)10-23-26-11-12-1-6-15(21)16(22)9-12/h1-10H,11H2/b23-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CAR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human LXR-beta |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human LXR-beta |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PPAR-alpha |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099491
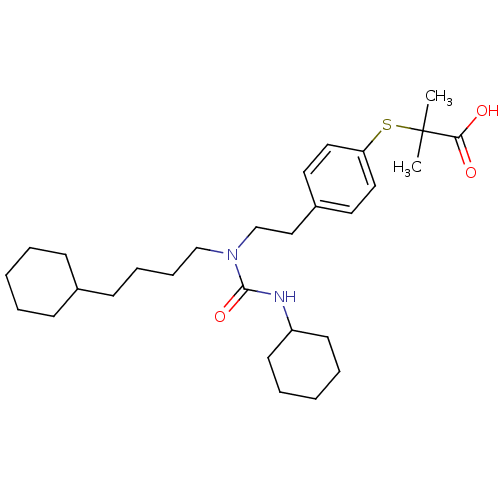
(2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PPAR-alpha |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PPAR-delta |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127222
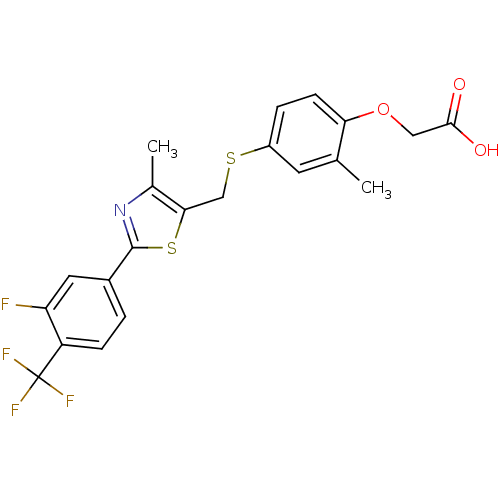
(CHEMBL38508 | GW-0742 | {4-[2-(3-Fluoro-4-trifluor...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(c(F)c1)C(F)(F)F Show InChI InChI=1S/C21H17F4NO3S2/c1-11-7-14(4-6-17(11)29-9-19(27)28)30-10-18-12(2)26-20(31-18)13-3-5-15(16(22)8-13)21(23,24)25/h3-8H,9-10H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PPAR-delta |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PPAR-gamma |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085048
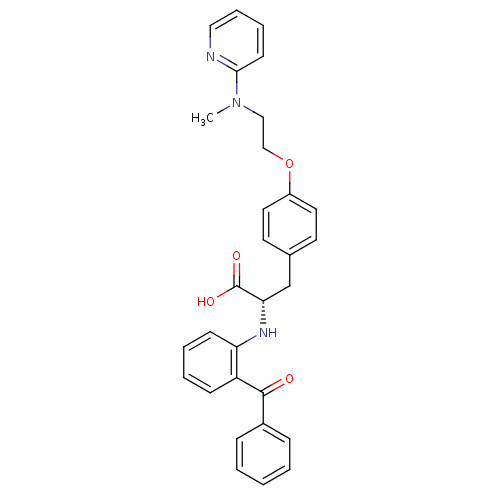
((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(methyl-pyri...)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1ccccn1 Show InChI InChI=1S/C30H29N3O4/c1-33(28-13-7-8-18-31-28)19-20-37-24-16-14-22(15-17-24)21-27(30(35)36)32-26-12-6-5-11-25(26)29(34)23-9-3-2-4-10-23/h2-18,27,32H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PPAR-gamma |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PXR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50030477
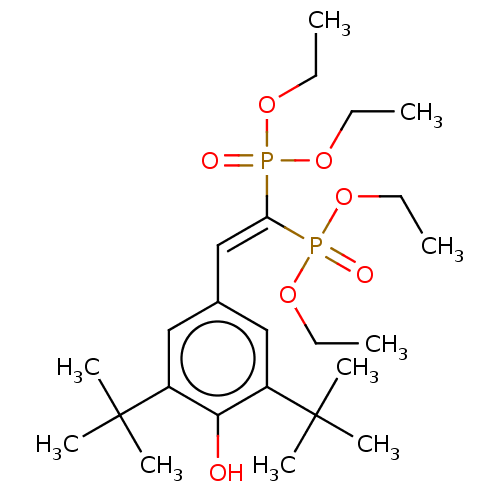
(CHEBI:77317 | CHEMBL458767 | SR-12813)Show SMILES [#6]-[#6]-[#8]P(=O)([#8]-[#6]-[#6])[#6](=[#6]\c1cc(c(-[#8])c(c1)C([#6])([#6])[#6])C([#6])([#6])[#6])\P(=O)([#8]-[#6]-[#6])[#8]-[#6]-[#6] Show InChI InChI=1S/C24H42O7P2/c1-11-28-32(26,29-12-2)21(33(27,30-13-3)31-14-4)17-18-15-19(23(5,6)7)22(25)20(16-18)24(8,9)10/h15-17,25H,11-14H2,1-10H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PXR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human RXR-alpha |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human RXR-alpha |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human VDR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human VDR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human ER |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human ER |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM8903
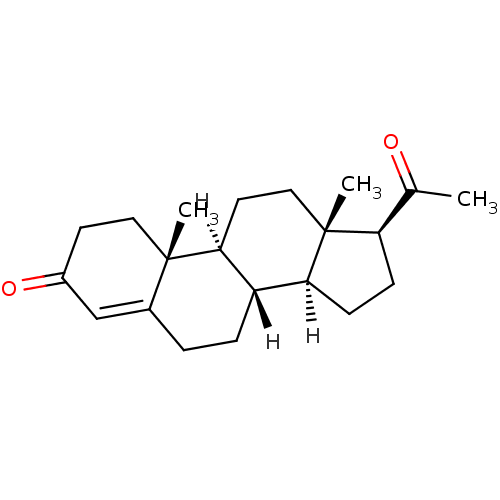
((1S,2R,10S,11S,14S,15S)-14-acetyl-2,15-dimethyltet...)Show SMILES [H][C@@]12CC[C@H](C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:20| Show InChI InChI=1S/C21H30O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h12,16-19H,4-11H2,1-3H3/t16-,17+,18-,19-,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human TR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50550152

(CHEMBL4758064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])[C@@H](O)C[C@]12C)[C@H](C)CCC(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human GR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data