 Found 593 hits with Last Name = 'fitzek' and Initial = 'm'
Found 593 hits with Last Name = 'fitzek' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070262
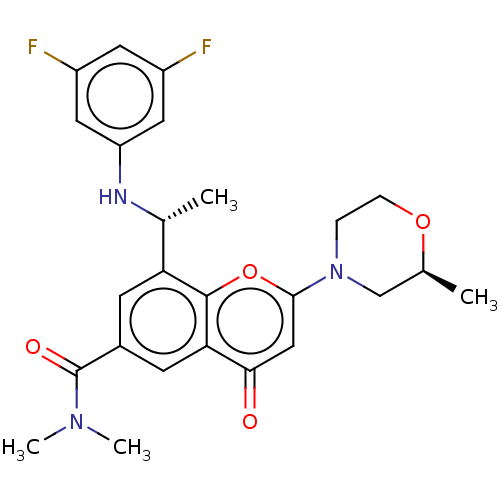
(CHEMBL3408270 | US9718800, 9.02b)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCO[C@@H](C)C1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-14-13-30(5-6-33-14)23-12-22(31)21-8-16(25(32)29(3)4)7-20(24(21)34-23)15(2)28-19-10-17(26)9-18(27)11-19/h7-12,14-15,28H,5-6,13H2,1-4H3/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-null human MDA-MB-468 cells assessed as inhibition of Akt phosphorylation after 2 hrs |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7657
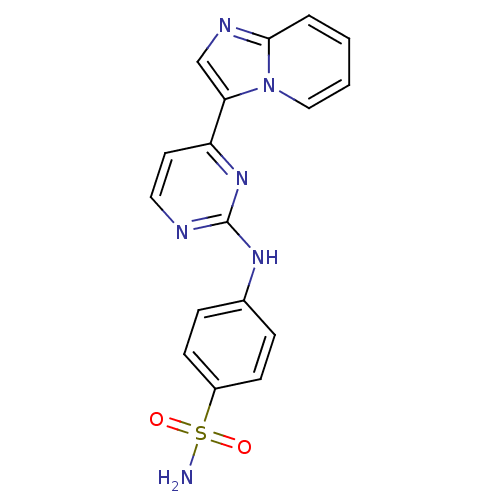
(4-[(4-{imidazo[1,2-a]pyridin-3-yl}pyrimidin-2-yl)a...)Show SMILES NS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccccn23)cc1 Show InChI InChI=1S/C17H14N6O2S/c18-26(24,25)13-6-4-12(5-7-13)21-17-19-9-8-14(22-17)15-11-20-16-3-1-2-10-23(15)16/h1-11H,(H2,18,24,25)(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 21: 4698-701 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.093
BindingDB Entry DOI: 10.7270/Q2GF0TVK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070324
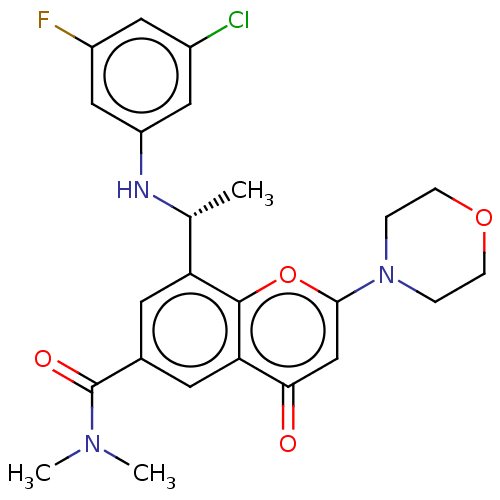
(CHEMBL3408252)Show SMILES C[C@@H](Nc1cc(F)cc(Cl)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C24H25ClFN3O4/c1-14(27-18-11-16(25)10-17(26)12-18)19-8-15(24(31)28(2)3)9-20-21(30)13-22(33-23(19)20)29-4-6-32-7-5-29/h8-14,27H,4-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-null human MDA-MB-468 cells assessed as inhibition of Akt phosphorylation after 2 hrs |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239117

(CHEMBL4060768)Show SMILES Fc1cc(F)cc(c1)N1CCC[C@@H]1c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H29F2N3O5/c29-19-14-20(30)16-21(15-19)33-3-1-2-24(33)22-12-18(28(35)32-6-10-37-11-7-32)13-23-25(34)17-26(38-27(22)23)31-4-8-36-9-5-31/h12-17,24H,1-11H2/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239123
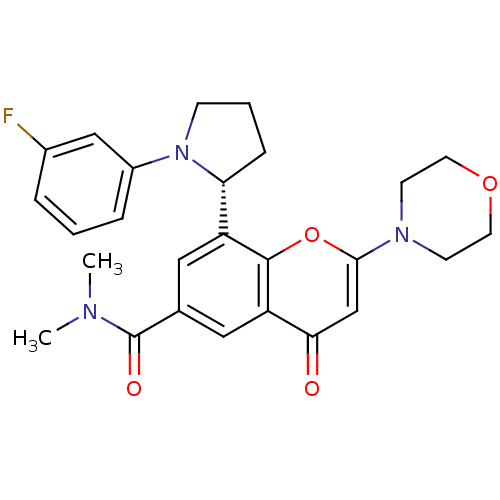
(CHEMBL4078233)Show SMILES CN(C)C(=O)c1cc([C@H]2CCCN2c2cccc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 |r| Show InChI InChI=1S/C26H28FN3O4/c1-28(2)26(32)17-13-20(22-7-4-8-30(22)19-6-3-5-18(27)15-19)25-21(14-17)23(31)16-24(34-25)29-9-11-33-12-10-29/h3,5-6,13-16,22H,4,7-12H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239122

(CHEMBL4094771)Show SMILES CN(C)C(=O)c1cc(C2CCCN2c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C26H27F2N3O4/c1-29(2)26(33)16-10-20(22-4-3-5-31(22)19-13-17(27)12-18(28)14-19)25-21(11-16)23(32)15-24(35-25)30-6-8-34-9-7-30/h10-15,22H,3-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239120
(CHEMBL4101005)Show SMILES Fc1cc(F)cc(c1)N1CCC[C@@H]1c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N1CC1 |r| Show InChI InChI=1S/C26H25F2N3O4/c27-17-12-18(28)14-19(13-17)31-3-1-2-22(31)20-10-16(26(33)30-4-5-30)11-21-23(32)15-24(35-25(20)21)29-6-8-34-9-7-29/h10-15,22H,1-9H2/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500920
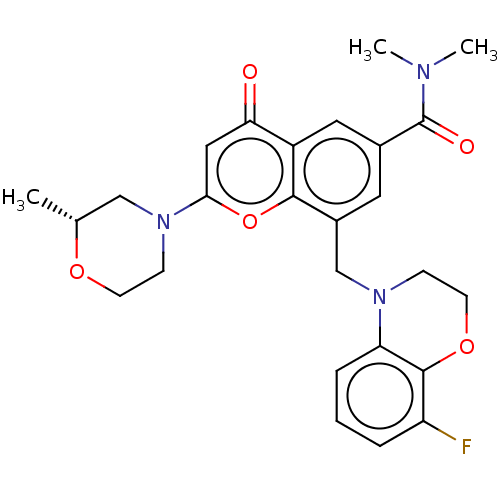
(CHEMBL3798914)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(8-9-33-16)23-13-22(31)19-12-17(26(32)28(2)3)11-18(24(19)35-23)15-29-7-10-34-25-20(27)5-4-6-21(25)29/h4-6,11-13,16H,7-10,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM119283
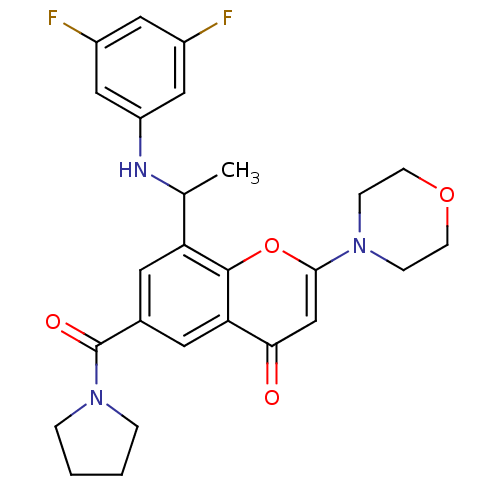
(US8673906, 4.02 | US9718800, 4.02)Show SMILES CC(Nc1cc(F)cc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N1CCCC1 Show InChI InChI=1S/C26H27F2N3O4/c1-16(29-20-13-18(27)12-19(28)14-20)21-10-17(26(33)31-4-2-3-5-31)11-22-23(32)15-24(35-25(21)22)30-6-8-34-9-7-30/h10-16,29H,2-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-null human MDA-MB-468 cells assessed as inhibition of Akt phosphorylation after 2 hrs |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239126
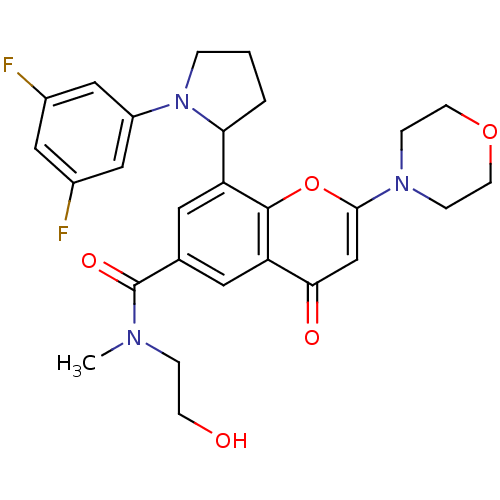
(CHEMBL4067312)Show SMILES CN(CCO)C(=O)c1cc(C2CCCN2c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C27H29F2N3O5/c1-30(5-8-33)27(35)17-11-21(23-3-2-4-32(23)20-14-18(28)13-19(29)15-20)26-22(12-17)24(34)16-25(37-26)31-6-9-36-10-7-31/h11-16,23,33H,2-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500917
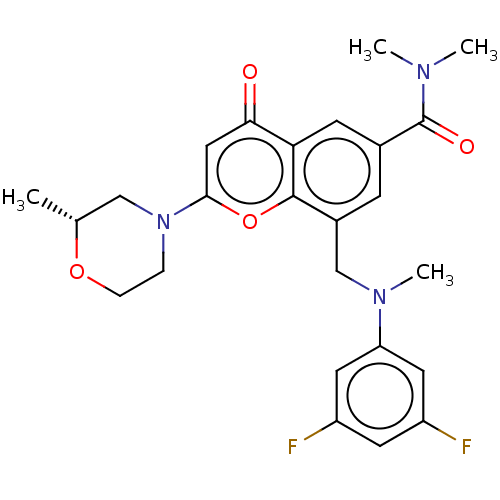
(CHEMBL3797698)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN(C)c3cc(F)cc(F)c3)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-15-13-30(5-6-33-15)23-12-22(31)21-8-16(25(32)28(2)3)7-17(24(21)34-23)14-29(4)20-10-18(26)9-19(27)11-20/h7-12,15H,5-6,13-14H2,1-4H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048722

(CHEMBL3319646)Show SMILES CC(Nc1cccc(F)c1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C25H31FN6O3/c1-17(28-20-6-4-5-19(26)14-20)21-13-18(25(34)27-7-8-30(2)3)16-32-23(33)15-22(29-24(21)32)31-9-11-35-12-10-31/h4-6,13-17,28H,7-12H2,1-3H3,(H,27,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070327
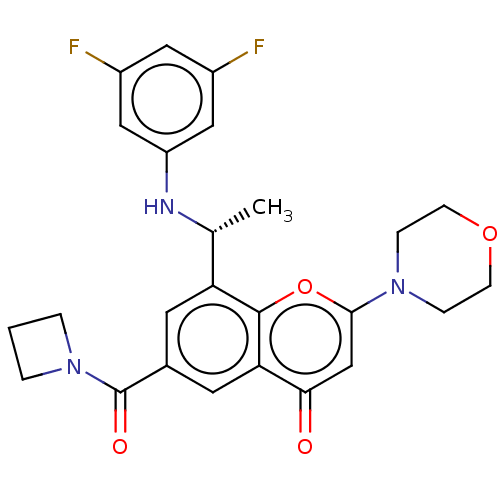
(CHEMBL3408262)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N1CCC1 |r| Show InChI InChI=1S/C25H25F2N3O4/c1-15(28-19-12-17(26)11-18(27)13-19)20-9-16(25(32)30-3-2-4-30)10-21-22(31)14-23(34-24(20)21)29-5-7-33-8-6-29/h9-15,28H,2-8H2,1H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-null human MDA-MB-468 cells assessed as inhibition of Akt phosphorylation after 2 hrs |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239121
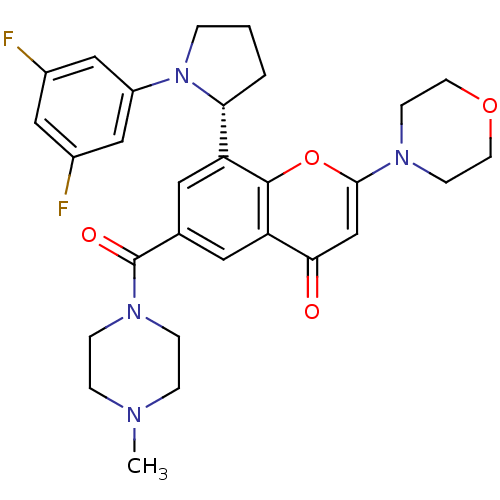
(CHEMBL4082650)Show SMILES CN1CCN(CC1)C(=O)c1cc([C@H]2CCCN2c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 |r| Show InChI InChI=1S/C29H32F2N4O4/c1-32-5-7-34(8-6-32)29(37)19-13-23(25-3-2-4-35(25)22-16-20(30)15-21(31)17-22)28-24(14-19)26(36)18-27(39-28)33-9-11-38-12-10-33/h13-18,25H,2-12H2,1H3/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239118
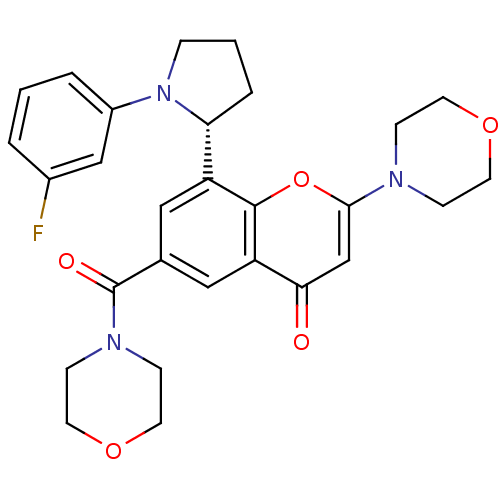
(CHEMBL4090811)Show SMILES Fc1cccc(c1)N1CCC[C@@H]1c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H30FN3O5/c29-20-3-1-4-21(17-20)32-6-2-5-24(32)22-15-19(28(34)31-9-13-36-14-10-31)16-23-25(33)18-26(37-27(22)23)30-7-11-35-12-8-30/h1,3-4,15-18,24H,2,5-14H2/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500918
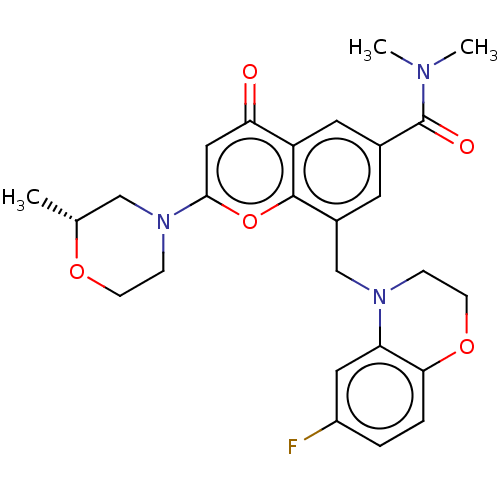
(CHEMBL3799511)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4ccc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(7-8-33-16)24-13-22(31)20-11-17(26(32)28(2)3)10-18(25(20)35-24)15-29-6-9-34-23-5-4-19(27)12-21(23)29/h4-5,10-13,16H,6-9,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500922
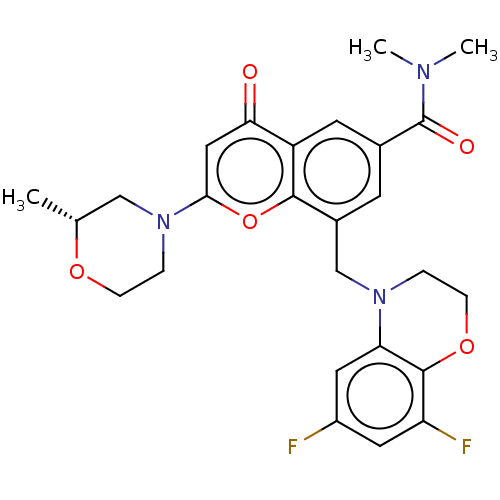
(CHEMBL3797812)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H27F2N3O5/c1-15-13-31(5-6-34-15)23-12-22(32)19-9-16(26(33)29(2)3)8-17(24(19)36-23)14-30-4-7-35-25-20(28)10-18(27)11-21(25)30/h8-12,15H,4-7,13-14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50349773
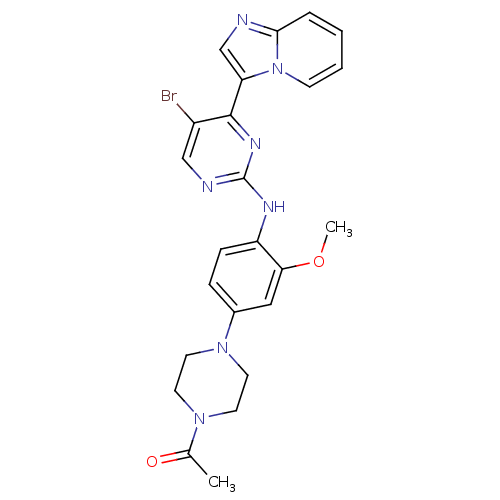
(CHEMBL1809194)Show SMILES COc1cc(ccc1Nc1ncc(Br)c(n1)-c1cnc2ccccn12)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C24H24BrN7O2/c1-16(33)30-9-11-31(12-10-30)17-6-7-19(21(13-17)34-2)28-24-27-14-18(25)23(29-24)20-15-26-22-5-3-4-8-32(20)22/h3-8,13-15H,9-12H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 21: 4698-701 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.093
BindingDB Entry DOI: 10.7270/Q2GF0TVK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239125
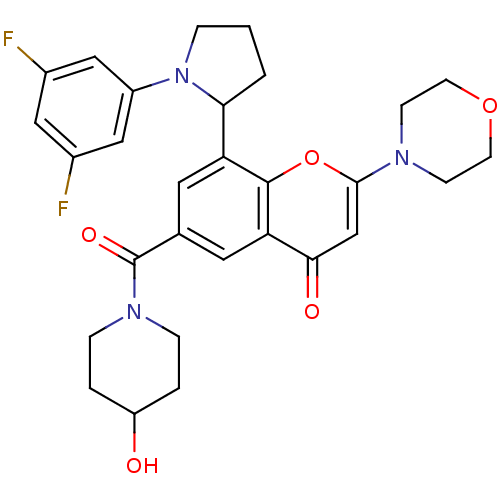
(CHEMBL4083021)Show SMILES OC1CCN(CC1)C(=O)c1cc(C2CCCN2c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C29H31F2N3O5/c30-19-14-20(31)16-21(15-19)34-5-1-2-25(34)23-12-18(29(37)33-6-3-22(35)4-7-33)13-24-26(36)17-27(39-28(23)24)32-8-10-38-11-9-32/h12-17,22,25,35H,1-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048713
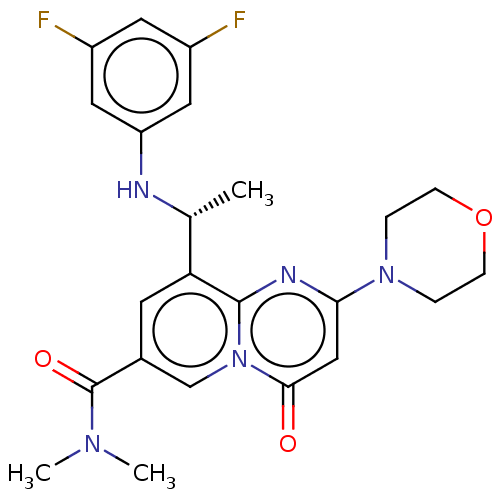
(CHEMBL3319490)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C23H25F2N5O3/c1-14(26-18-10-16(24)9-17(25)11-18)19-8-15(23(32)28(2)3)13-30-21(31)12-20(27-22(19)30)29-4-6-33-7-5-29/h8-14,26H,4-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070210
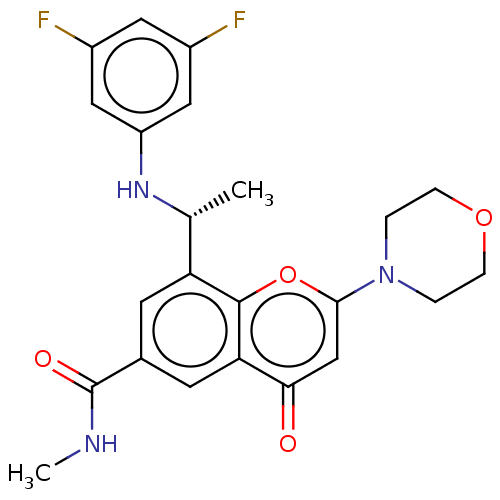
(CHEMBL3408267)Show SMILES CNC(=O)c1cc([C@@H](C)Nc2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 |r| Show InChI InChI=1S/C23H23F2N3O4/c1-13(27-17-10-15(24)9-16(25)11-17)18-7-14(23(30)26-2)8-19-20(29)12-21(32-22(18)19)28-3-5-31-6-4-28/h7-13,27H,3-6H2,1-2H3,(H,26,30)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-null human MDA-MB-468 cells assessed as inhibition of Akt phosphorylation after 2 hrs |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070322

(CHEMBL3408248 | US9718800, 3.06b)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C24H25F2N3O4/c1-14(27-18-11-16(25)10-17(26)12-18)19-8-15(24(31)28(2)3)9-20-21(30)13-22(33-23(19)20)29-4-6-32-7-5-29/h8-14,27H,4-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-null human MDA-MB-468 cells assessed as inhibition of Akt phosphorylation after 2 hrs |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239127
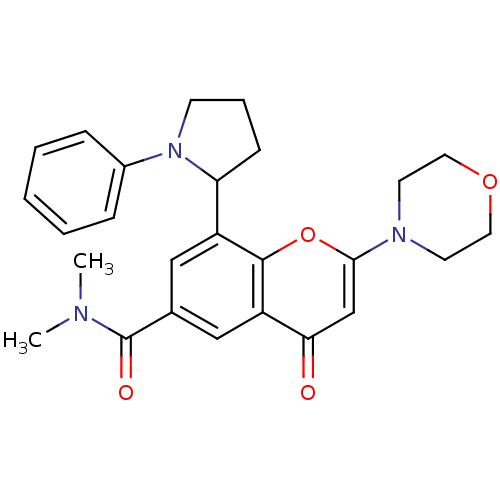
(CHEMBL4086125)Show SMILES CN(C)C(=O)c1cc(C2CCCN2c2ccccc2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C26H29N3O4/c1-27(2)26(31)18-15-20(22-9-6-10-29(22)19-7-4-3-5-8-19)25-21(16-18)23(30)17-24(33-25)28-11-13-32-14-12-28/h3-5,7-8,15-17,22H,6,9-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239124
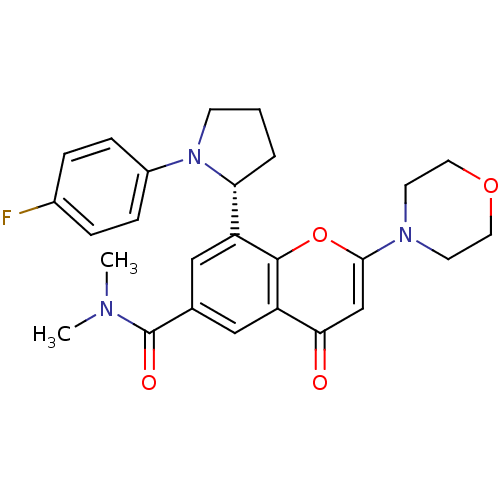
(CHEMBL4102511)Show SMILES CN(C)C(=O)c1cc([C@H]2CCCN2c2ccc(F)cc2)c2oc(cc(=O)c2c1)N1CCOCC1 |r| Show InChI InChI=1S/C26H28FN3O4/c1-28(2)26(32)17-14-20(22-4-3-9-30(22)19-7-5-18(27)6-8-19)25-21(15-17)23(31)16-24(34-25)29-10-12-33-13-11-29/h5-8,14-16,22H,3-4,9-13H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070327

(CHEMBL3408262)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N1CCC1 |r| Show InChI InChI=1S/C25H25F2N3O4/c1-15(28-19-12-17(26)11-18(27)13-19)20-9-16(25(32)30-3-2-4-30)10-21-22(31)14-23(34-24(20)21)29-5-7-33-8-6-29/h9-15,28H,2-8H2,1H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 by Kinase-Glo Plus assay |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500924
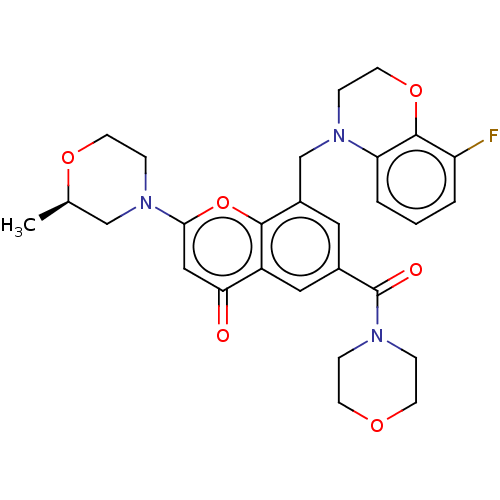
(CHEMBL3799158)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H30FN3O6/c1-18-16-32(8-11-36-18)25-15-24(33)21-14-19(28(34)30-5-9-35-10-6-30)13-20(26(21)38-25)17-31-7-12-37-27-22(29)3-2-4-23(27)31/h2-4,13-15,18H,5-12,16-17H2,1H3/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
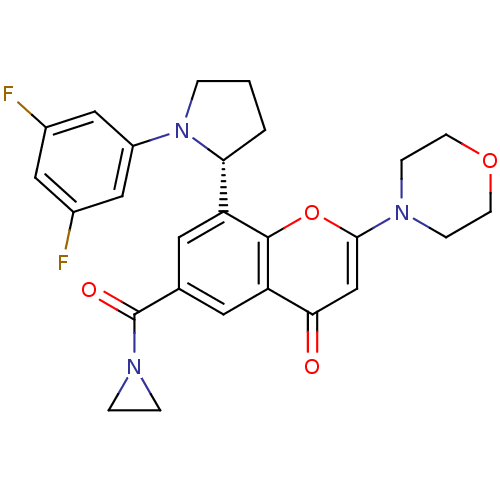
(Homo sapiens (Human)) | BDBM50070262

(CHEMBL3408270 | US9718800, 9.02b)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCO[C@@H](C)C1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-14-13-30(5-6-33-14)23-12-22(31)21-8-16(25(32)29(3)4)7-20(24(21)34-23)15(2)28-19-10-17(26)9-18(27)11-19/h7-12,14-15,28H,5-6,13H2,1-4H3/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 by Kinase-Glo Plus assay |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070322

(CHEMBL3408248 | US9718800, 3.06b)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C24H25F2N3O4/c1-14(27-18-11-16(25)10-17(26)12-18)19-8-15(24(31)28(2)3)9-20-21(30)13-22(33-23(19)20)29-4-6-32-7-5-29/h8-14,27H,4-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 by Kinase-Glo Plus assay |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048710
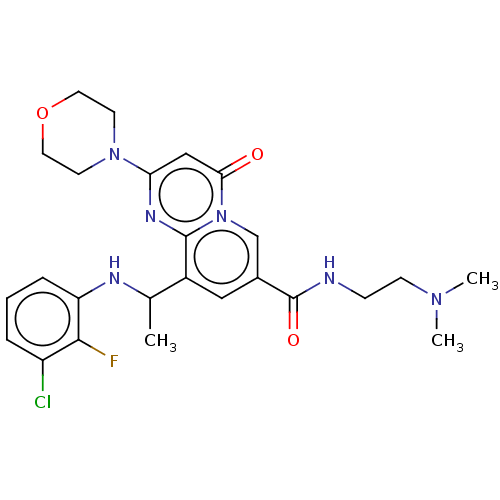
(CHEMBL3319485)Show SMILES CC(Nc1cccc(Cl)c1F)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C25H30ClFN6O3/c1-16(29-20-6-4-5-19(26)23(20)27)18-13-17(25(35)28-7-8-31(2)3)15-33-22(34)14-21(30-24(18)33)32-9-11-36-12-10-32/h4-6,13-16,29H,7-12H2,1-3H3,(H,28,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239119
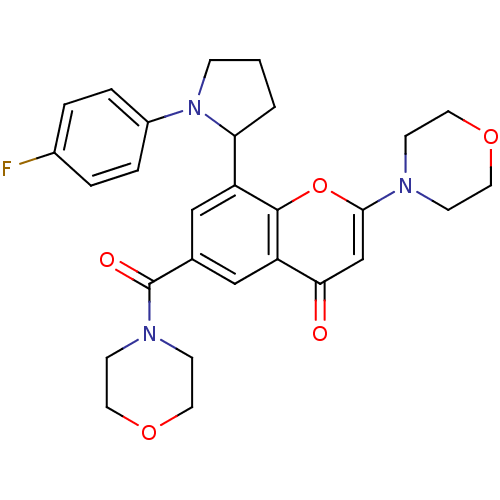
(CHEMBL4062879)Show SMILES Fc1ccc(cc1)N1CCCC1c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N1CCOCC1 Show InChI InChI=1S/C28H30FN3O5/c29-20-3-5-21(6-4-20)32-7-1-2-24(32)22-16-19(28(34)31-10-14-36-15-11-31)17-23-25(33)18-26(37-27(22)23)30-8-12-35-13-9-30/h3-6,16-18,24H,1-2,7-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrs |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500929
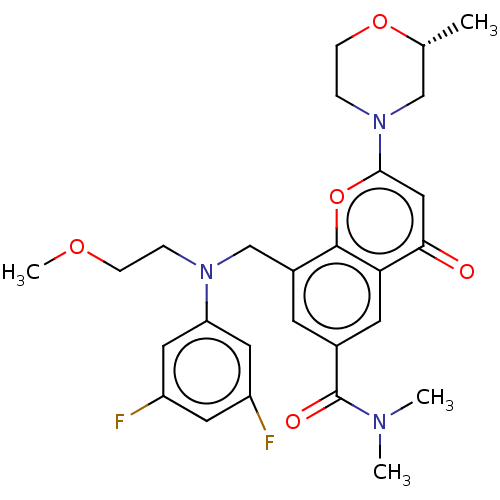
(CHEMBL3800231)Show SMILES COCCN(Cc1cc(cc2c1oc(cc2=O)N1CCO[C@H](C)C1)C(=O)N(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C27H31F2N3O5/c1-17-15-32(6-8-36-17)25-14-24(33)23-10-18(27(34)30(2)3)9-19(26(23)37-25)16-31(5-7-35-4)22-12-20(28)11-21(29)13-22/h9-14,17H,5-8,15-16H2,1-4H3/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50253249
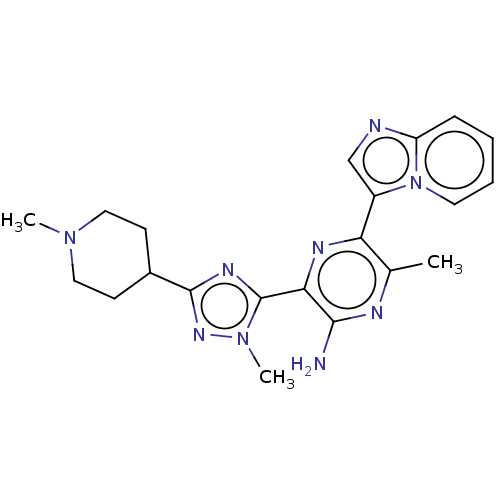
(CHEMBL4093351)Show SMILES CN1CCC(CC1)c1nc(-c2nc(-c3cnc4ccccn34)c(C)nc2N)n(C)n1 Show InChI InChI=1S/C21H25N9/c1-13-17(15-12-23-16-6-4-5-9-30(15)16)25-18(19(22)24-13)21-26-20(27-29(21)3)14-7-10-28(2)11-8-14/h4-6,9,12,14H,7-8,10-11H2,1-3H3,(H2,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 27: 3030-3035 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.028
BindingDB Entry DOI: 10.7270/Q23R0W9X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500917

(CHEMBL3797698)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN(C)c3cc(F)cc(F)c3)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-15-13-30(5-6-33-15)23-12-22(31)21-8-16(25(32)28(2)3)7-17(24(21)34-23)14-29(4)20-10-18(26)9-19(27)11-20/h7-12,15H,5-6,13-14H2,1-4H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048713

(CHEMBL3319490)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C23H25F2N5O3/c1-14(26-18-10-16(24)9-17(25)11-18)19-8-15(23(32)28(2)3)13-30-21(31)12-20(27-22(19)30)29-4-6-33-7-5-29/h8-14,26H,4-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta assessed as depletion of ATP substrate by Ultra Glo luciferase assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070324

(CHEMBL3408252)Show SMILES C[C@@H](Nc1cc(F)cc(Cl)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C24H25ClFN3O4/c1-14(27-18-11-16(25)10-17(26)12-18)19-8-15(24(31)28(2)3)9-20-21(30)13-22(33-23(19)20)29-4-6-32-7-5-29/h8-14,27H,4-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 by Kinase-Glo Plus assay |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070323
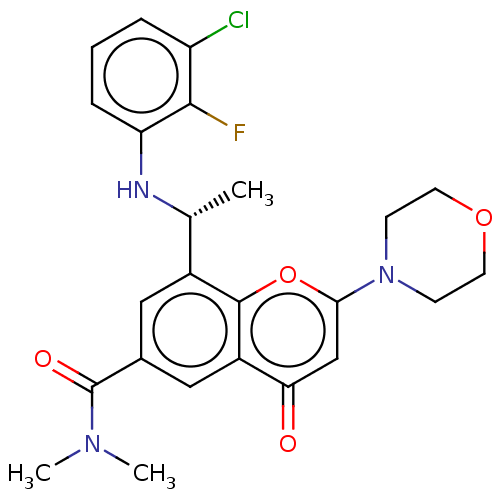
(CHEMBL3408250 | US9718800, 3.04b)Show SMILES C[C@@H](Nc1cccc(Cl)c1F)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C24H25ClFN3O4/c1-14(27-19-6-4-5-18(25)22(19)26)16-11-15(24(31)28(2)3)12-17-20(30)13-21(33-23(16)17)29-7-9-32-10-8-29/h4-6,11-14,27H,7-10H2,1-3H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 by Kinase-Glo Plus assay |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070325
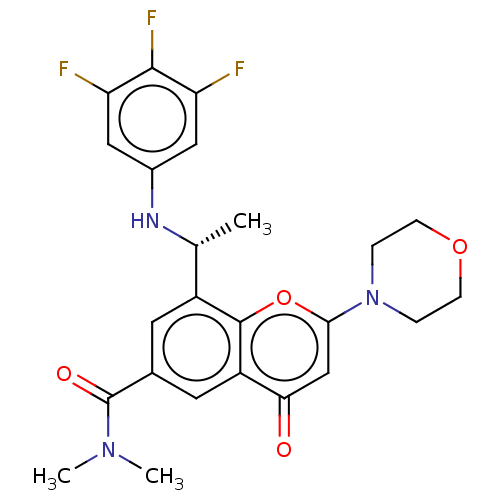
(CHEMBL3408256)Show SMILES C[C@@H](Nc1cc(F)c(F)c(F)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C24H24F3N3O4/c1-13(28-15-10-18(25)22(27)19(26)11-15)16-8-14(24(32)29(2)3)9-17-20(31)12-21(34-23(16)17)30-4-6-33-7-5-30/h8-13,28H,4-7H2,1-3H3/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 by Kinase-Glo Plus assay |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239118

(CHEMBL4090811)Show SMILES Fc1cccc(c1)N1CCC[C@@H]1c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H30FN3O5/c29-20-3-1-4-21(17-20)32-6-2-5-24(32)22-15-19(28(34)31-9-13-36-14-10-31)16-23-25(33)18-26(37-27(22)23)30-7-11-35-12-8-30/h1,3-4,15-18,24H,2,5-14H2/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranes |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500915
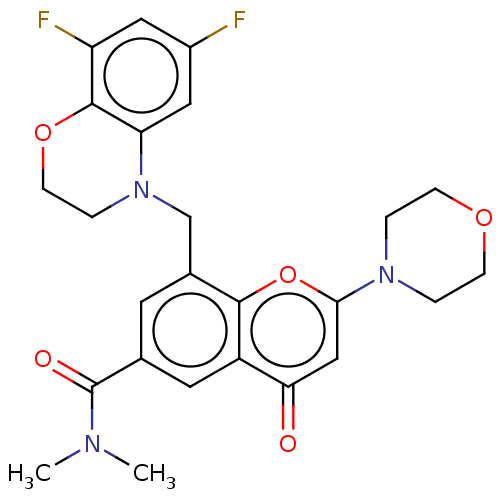
(CHEMBL3797750)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H25F2N3O5/c1-28(2)25(32)15-9-16(14-30-5-8-34-24-19(27)11-17(26)12-20(24)30)23-18(10-15)21(31)13-22(35-23)29-3-6-33-7-4-29/h9-13H,3-8,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500920

(CHEMBL3798914)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(8-9-33-16)23-13-22(31)19-12-17(26(32)28(2)3)11-18(24(19)35-23)15-29-7-10-34-25-20(27)5-4-6-21(25)29/h4-6,11-13,16H,7-10,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070326
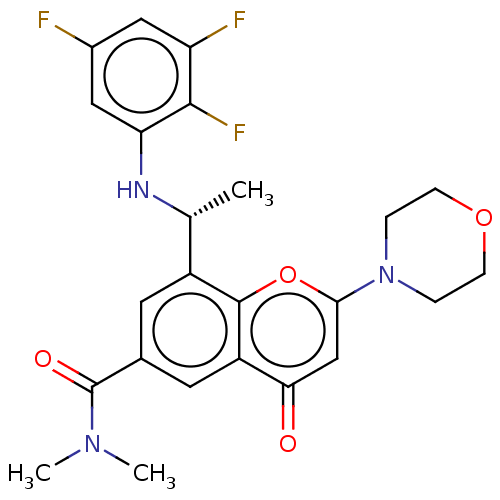
(CHEMBL3408257 | US9718800, 3.13b)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1F)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C24H24F3N3O4/c1-13(28-19-11-15(25)10-18(26)22(19)27)16-8-14(24(32)29(2)3)9-17-20(31)12-21(34-23(16)17)30-4-6-33-7-5-30/h8-13,28H,4-7H2,1-3H3/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-null human MDA-MB-468 cells assessed as inhibition of Akt phosphorylation after 2 hrs |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50253251
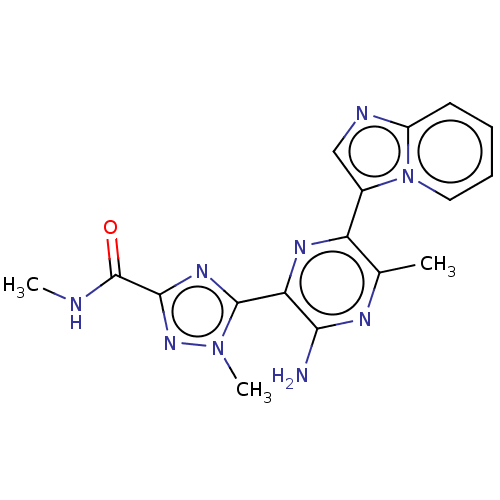
(CHEMBL4072637)Show SMILES CNC(=O)c1nc(-c2nc(-c3cnc4ccccn34)c(C)nc2N)n(C)n1 Show InChI InChI=1S/C17H17N9O/c1-9-12(10-8-20-11-6-4-5-7-26(10)11)22-13(14(18)21-9)16-23-15(17(27)19-2)24-25(16)3/h4-8H,1-3H3,(H2,18,21)(H,19,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 27: 3030-3035 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.028
BindingDB Entry DOI: 10.7270/Q23R0W9X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500922

(CHEMBL3797812)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H27F2N3O5/c1-15-13-31(5-6-34-15)23-12-22(32)19-9-16(26(33)29(2)3)8-17(24(19)36-23)14-30-4-7-35-25-20(28)10-18(27)11-21(25)30/h8-12,15H,4-7,13-14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070210

(CHEMBL3408267)Show SMILES CNC(=O)c1cc([C@@H](C)Nc2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 |r| Show InChI InChI=1S/C23H23F2N3O4/c1-13(27-17-10-15(24)9-16(25)11-17)18-7-14(23(30)26-2)8-19-20(29)12-21(32-22(18)19)28-3-5-31-6-4-28/h7-13,27H,3-6H2,1-2H3,(H,26,30)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 by Kinase-Glo Plus assay |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500925
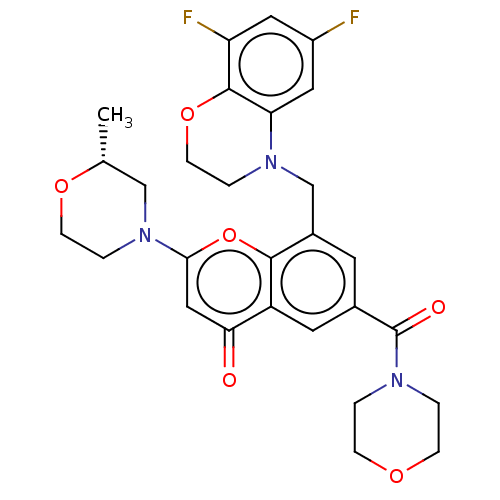
(CHEMBL3799673)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H29F2N3O6/c1-17-15-33(5-8-37-17)25-14-24(34)21-11-18(28(35)31-2-6-36-7-3-31)10-19(26(21)39-25)16-32-4-9-38-27-22(30)12-20(29)13-23(27)32/h10-14,17H,2-9,15-16H2,1H3/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50094479
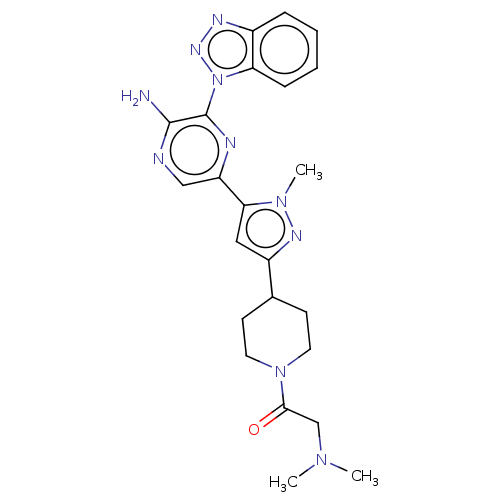
(CHEMBL3590225)Show SMILES CN(C)CC(=O)N1CCC(CC1)c1cc(-c2cnc(N)c(n2)-n2nnc3ccccc23)n(C)n1 Show InChI InChI=1S/C23H28N10O/c1-30(2)14-21(34)32-10-8-15(9-11-32)17-12-20(31(3)28-17)18-13-25-22(24)23(26-18)33-19-7-5-4-6-16(19)27-29-33/h4-7,12-13,15H,8-11,14H2,1-3H3,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3Kalpha using PIP2/ATP as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins ... |
Bioorg Med Chem Lett 25: 2679-85 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.084
BindingDB Entry DOI: 10.7270/Q2QJ7K1S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070318
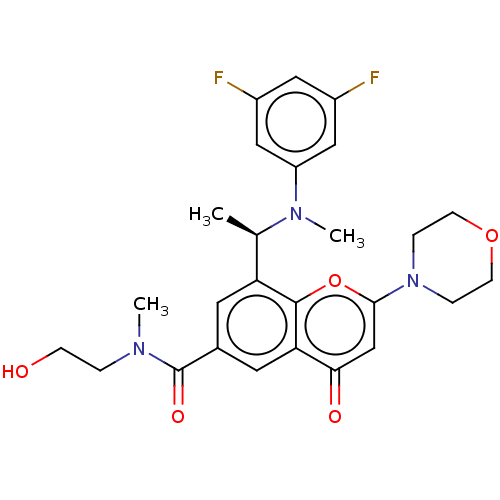
(CHEMBL3408277 | US9718800, 7.0b)Show SMILES C[C@@H](N(C)c1cc(F)cc(F)c1)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)CCO |r| Show InChI InChI=1S/C26H29F2N3O5/c1-16(30(3)20-13-18(27)12-19(28)14-20)21-10-17(26(34)29(2)4-7-32)11-22-23(33)15-24(36-25(21)22)31-5-8-35-9-6-31/h10-16,32H,4-9H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 by Kinase-Glo Plus assay |
J Med Chem 58: 943-62 (2015)
Article DOI: 10.1021/jm501629p
BindingDB Entry DOI: 10.7270/Q2VT1TS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239118

(CHEMBL4090811)Show SMILES Fc1cccc(c1)N1CCC[C@@H]1c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H30FN3O5/c29-20-3-1-4-21(17-20)32-6-2-5-24(32)22-15-19(28(34)31-9-13-36-14-10-31)16-23-25(33)18-26(37-27(22)23)30-7-11-35-12-8-30/h1,3-4,15-18,24H,2,5-14H2/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in pr... |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50239124

(CHEMBL4102511)Show SMILES CN(C)C(=O)c1cc([C@H]2CCCN2c2ccc(F)cc2)c2oc(cc(=O)c2c1)N1CCOCC1 |r| Show InChI InChI=1S/C26H28FN3O4/c1-28(2)26(32)17-14-20(22-4-3-9-30(22)19-7-5-18(27)6-8-19)25-21(15-17)23(31)16-24(34-25)29-10-12-33-13-11-29/h5-8,14-16,22H,3-4,9-13H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in pr... |
Bioorg Med Chem Lett 27: 1949-1954 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.027
BindingDB Entry DOI: 10.7270/Q2SB47W8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500918

(CHEMBL3799511)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4ccc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(7-8-33-16)24-13-22(31)20-11-17(26(32)28(2)3)10-18(25(20)35-24)15-29-6-9-34-23-5-4-19(27)12-21(23)29/h4-5,10-13,16H,6-9,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data