

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM238895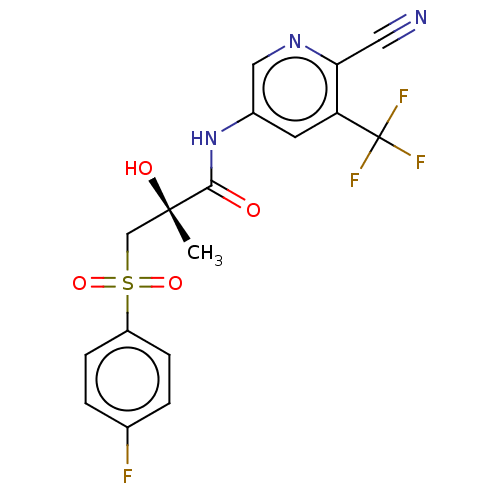 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239191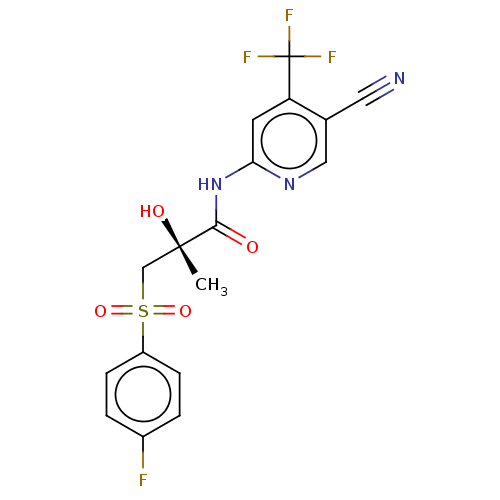 (US10053433, FC 3.077) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242618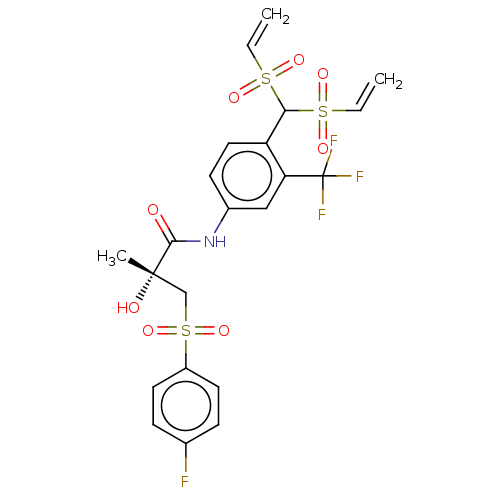 (US10053433, FC 4.025) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 580 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242588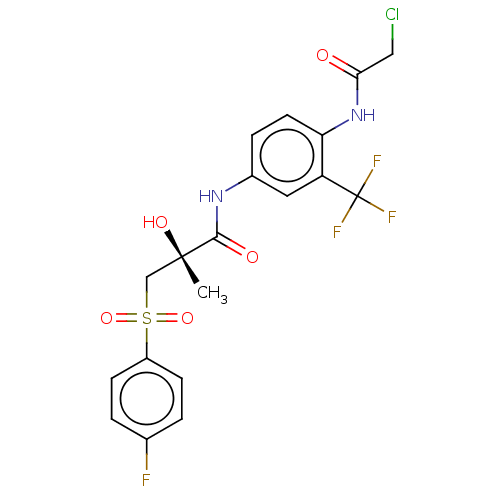 (US10053433, FC 4.039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 750 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Homo sapiens (Human)) | BDBM50253632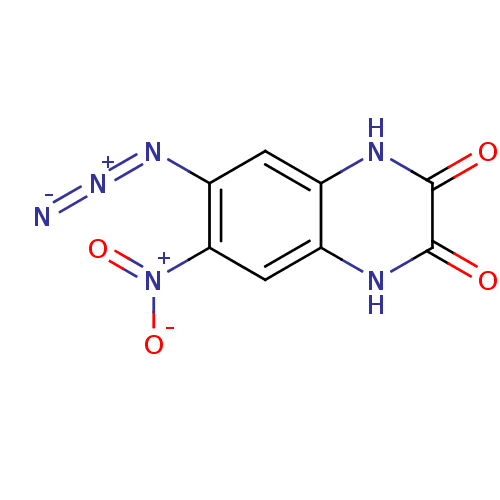 (6-Azido-7-nitro-1,4-dihydroquinoxaline-2,3-dione |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Inhibition of GluR2 receptor (unknown origin) | J Med Chem 51: 5856-60 (2008) Article DOI: 10.1021/jm701517b BindingDB Entry DOI: 10.7270/Q2W095R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242618 (US10053433, FC 4.025) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18825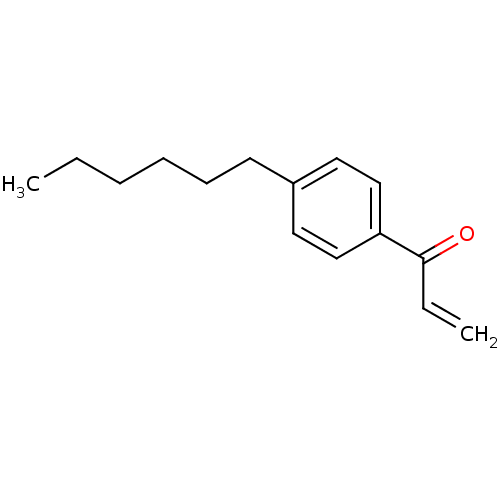 (1-(4-hexylphenyl)prop-2-en-1-one | Enone, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18814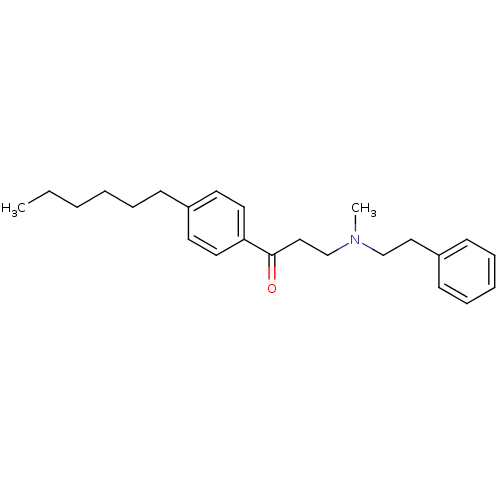 (1-(4-hexylphenyl)-3-[methyl(2-phenylethyl)amino]pr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239363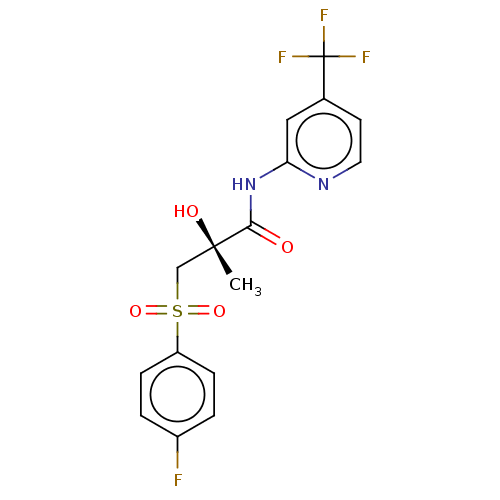 (US10053433, FC 4.126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18815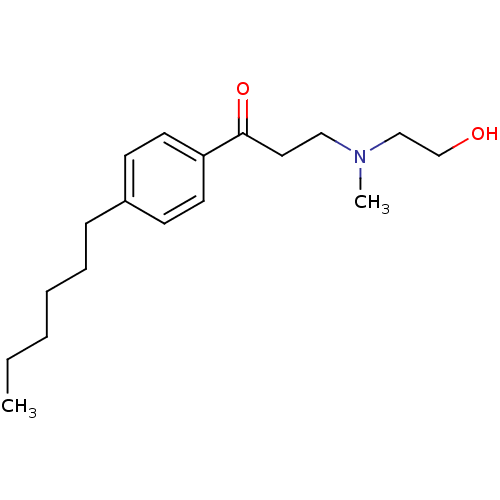 (1-(4-hexylphenyl)-3-[(2-hydroxyethyl)(methyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50425732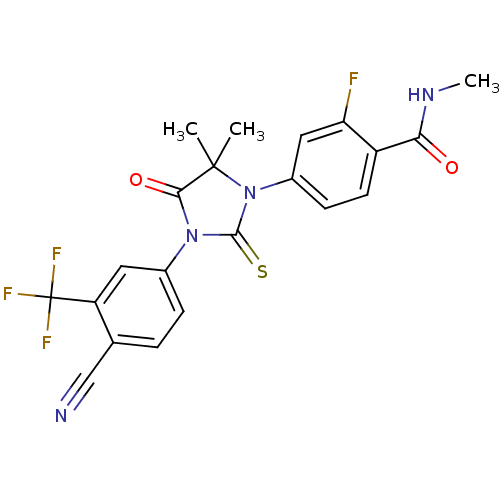 (ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank US Patent | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18816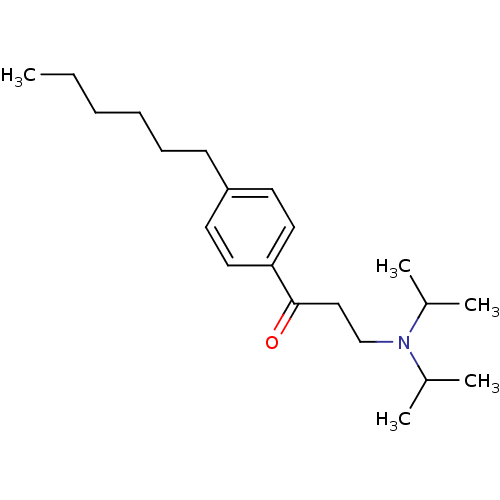 (3-[bis(propan-2-yl)amino]-1-(4-hexylphenyl)propan-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239316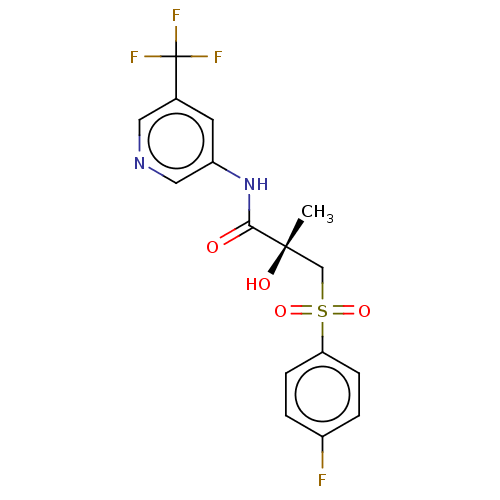 (US10053433, FC 4.116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18817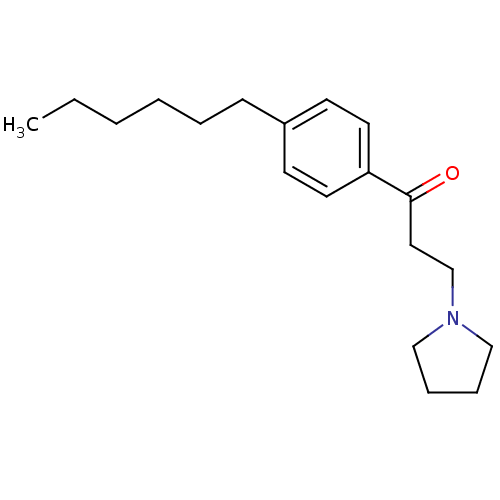 (1-(4-hexylphenyl)-3-(pyrrolidin-1-yl)propan-1-one ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18818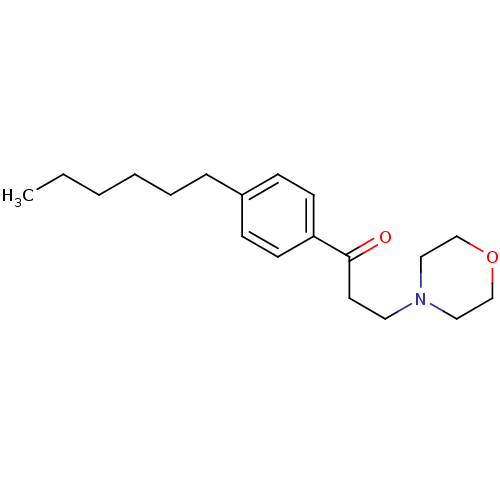 (1-(4-hexylphenyl)-3-(morpholin-4-yl)propan-1-one |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18815 (1-(4-hexylphenyl)-3-[(2-hydroxyethyl)(methyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18857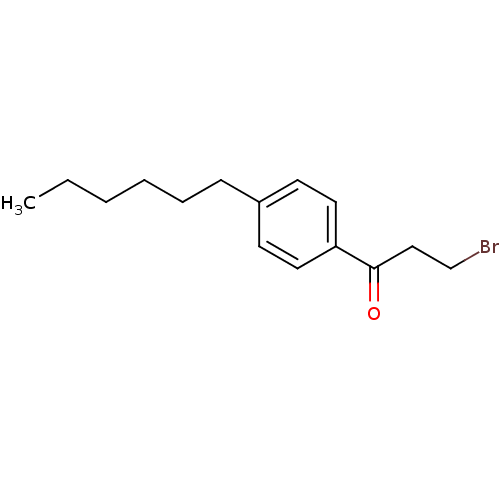 (3-bromo-1-(4-hexylphenyl)propan-1-one | beta-bromo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50094975 (956104-40-8 | ARN-509 | JNJ-56021927 | US10053433,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50094975 (956104-40-8 | ARN-509 | JNJ-56021927 | US10053433,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18814 (1-(4-hexylphenyl)-3-[methyl(2-phenylethyl)amino]pr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18819 (beta-Aminophenylketone, 3f | methyl 1-[3-(4-hexylp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18816 (3-[bis(propan-2-yl)amino]-1-(4-hexylphenyl)propan-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18818 (1-(4-hexylphenyl)-3-(morpholin-4-yl)propan-1-one |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18820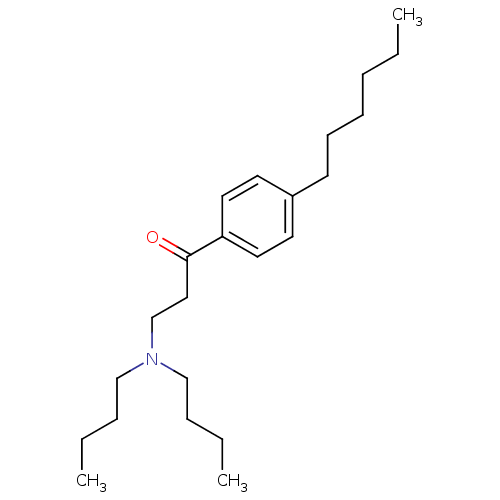 (3-(dibutylamino)-1-(4-hexylphenyl)propan-1-one | b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18857 (3-bromo-1-(4-hexylphenyl)propan-1-one | beta-bromo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18817 (1-(4-hexylphenyl)-3-(pyrrolidin-1-yl)propan-1-one ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18821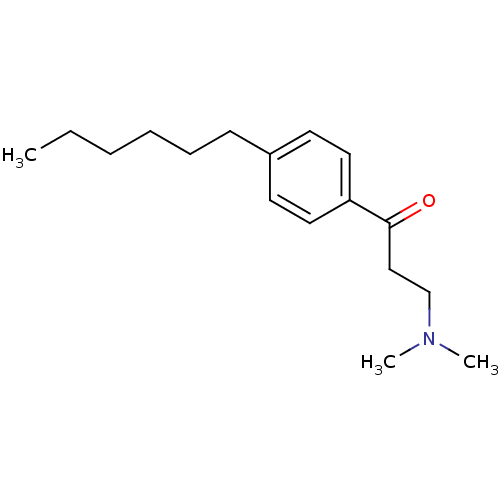 (3-(dimethylamino)-1-(4-hexylphenyl)propan-1-one | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18854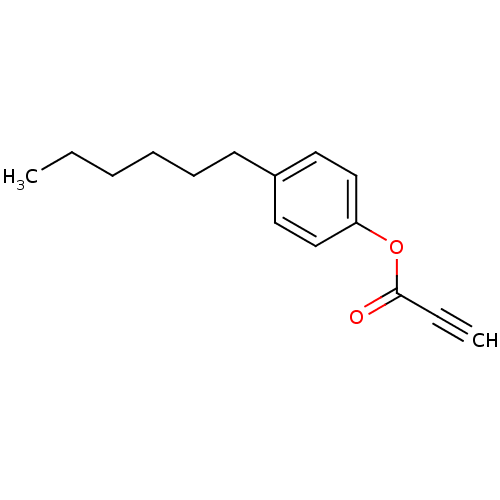 (4-hexylphenyl prop-2-ynoate | Propiolic acid deriv...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18819 (beta-Aminophenylketone, 3f | methyl 1-[3-(4-hexylp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18820 (3-(dibutylamino)-1-(4-hexylphenyl)propan-1-one | b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242578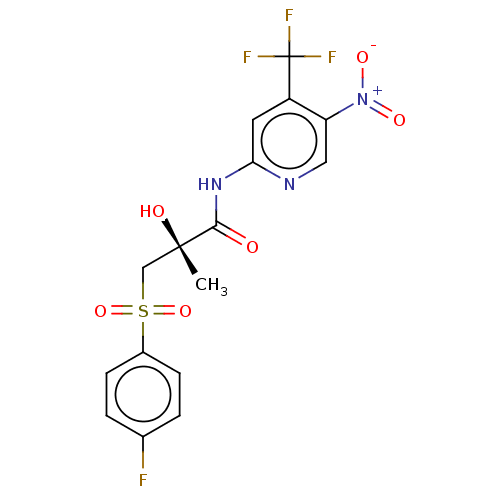 (US10053433, FC 4.127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.26E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18827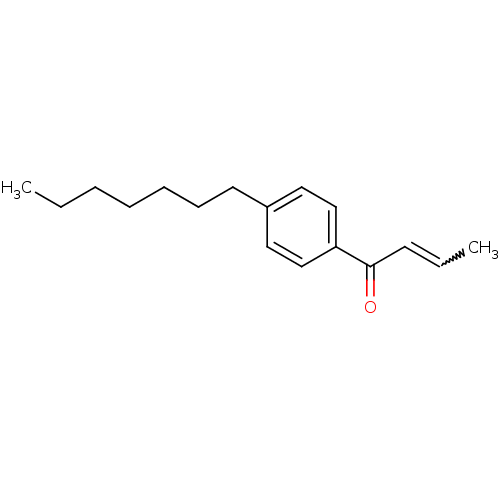 ((2E)-1-(4-heptylphenyl)but-2-en-1-one | Enone, 4b) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Homo sapiens (Human)) | BDBM50253650 (CHEMBL462490 | [1,2,5]oxadiazolo[3,4-g]quinoxaline...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Inhibition of GluR2 receptor (unknown origin) | J Med Chem 51: 5856-60 (2008) Article DOI: 10.1021/jm701517b BindingDB Entry DOI: 10.7270/Q2W095R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18843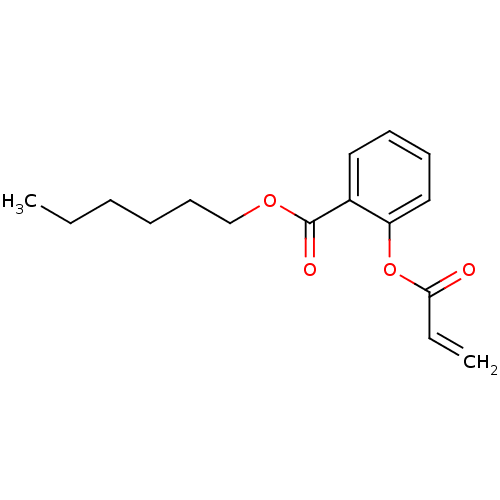 (Substituted Acrylate, 6k | hexyl 2-(prop-2-enoylox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50425732 (ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank US Patent | n/a | n/a | 8.61E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242578 (US10053433, FC 4.127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.74E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18838 (4-heptylphenyl prop-2-enoate | Substituted Acrylat...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18821 (3-(dimethylamino)-1-(4-hexylphenyl)propan-1-one | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18839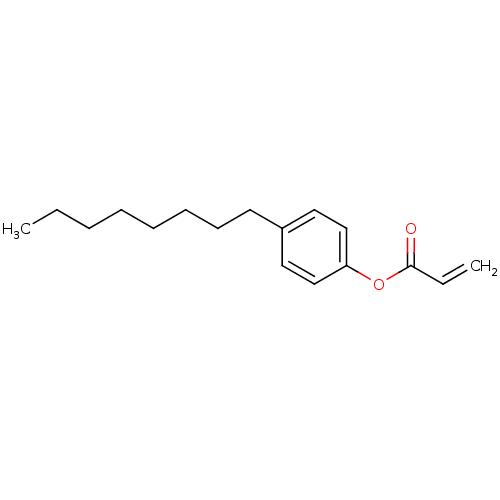 (4-octylphenyl prop-2-enoate | Substituted Acrylate...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18822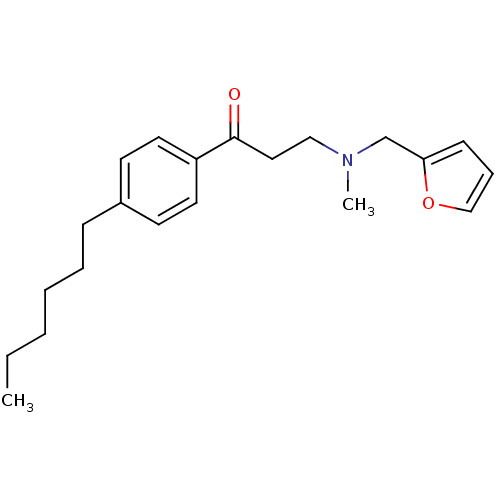 (3-[(furan-2-ylmethyl)(methyl)amino]-1-(4-hexylphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239363 (US10053433, FC 4.126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18823 (3-(dicyclohexylamino)-1-(4-hexylphenyl)propan-1-on...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18824 (1-(4-hexylphenyl)-3-(propylamino)propan-1-one | be...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18823 (3-(dicyclohexylamino)-1-(4-hexylphenyl)propan-1-on...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18854 (4-hexylphenyl prop-2-ynoate | Propiolic acid deriv...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha [148-410] (Homo sapiens (Human)) | BDBM18847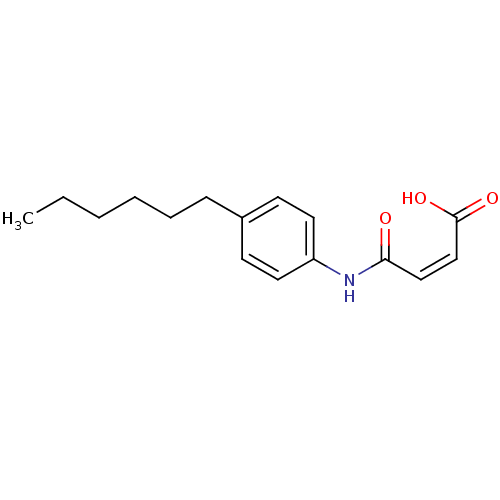 ((2Z)-3-[(4-hexylphenyl)carbamoyl]prop-2-enoic acid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta [209-461] (Homo sapiens (Human)) | BDBM18826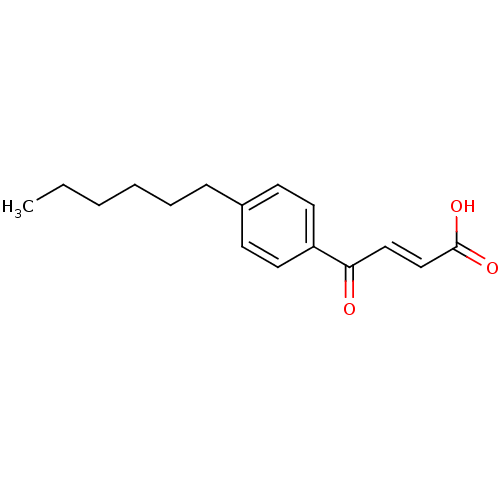 ((2E)-4-(4-hexylphenyl)-4-oxobut-2-enoic acid | Eno...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description IC50 is the concentration of each compound required to inhibit 50% of the binding between the TR LBD and the SRC2-2 peptide using fluorescence polari... | J Med Chem 50: 5269-5280 (2007) Article DOI: 10.1021/jm070556y BindingDB Entry DOI: 10.7270/Q24B2ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 115 total ) | Next | Last >> |