 Found 97 hits with Last Name = 'foekens' and Initial = 'ja'
Found 97 hits with Last Name = 'foekens' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228427
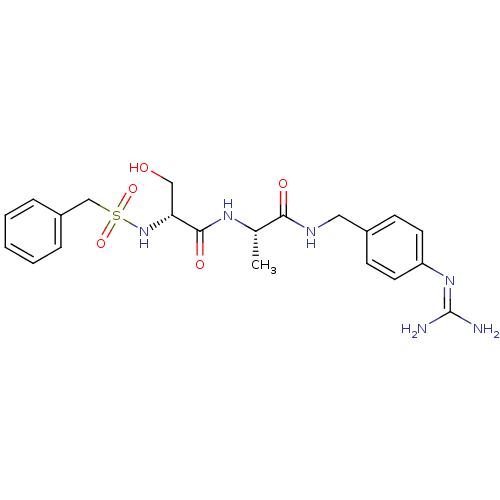
((R)-N-[(S)-1-(4-guanidino-benzylcarbamoyl)-ethyl]-...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C21H28N6O5S/c1-14(19(29)24-11-15-7-9-17(10-8-15)26-21(22)23)25-20(30)18(12-28)27-33(31,32)13-16-5-3-2-4-6-16/h2-10,14,18,27-28H,11-13H2,1H3,(H,24,29)(H,25,30)(H4,22,23,26)/t14-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228427

((R)-N-[(S)-1-(4-guanidino-benzylcarbamoyl)-ethyl]-...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C21H28N6O5S/c1-14(19(29)24-11-15-7-9-17(10-8-15)26-21(22)23)25-20(30)18(12-28)27-33(31,32)13-16-5-3-2-4-6-16/h2-10,14,18,27-28H,11-13H2,1H3,(H,24,29)(H,25,30)(H4,22,23,26)/t14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228422
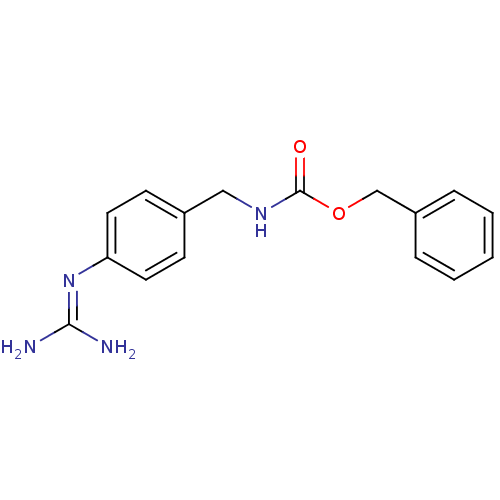
((4-guanidino-benzyl)-carbamic acid benzyl ester | ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C16H18N4O2/c17-15(18)20-14-8-6-12(7-9-14)10-19-16(21)22-11-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,19,21)(H4,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228427

((R)-N-[(S)-1-(4-guanidino-benzylcarbamoyl)-ethyl]-...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C21H28N6O5S/c1-14(19(29)24-11-15-7-9-17(10-8-15)26-21(22)23)25-20(30)18(12-28)27-33(31,32)13-16-5-3-2-4-6-16/h2-10,14,18,27-28H,11-13H2,1H3,(H,24,29)(H,25,30)(H4,22,23,26)/t14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228422

((4-guanidino-benzyl)-carbamic acid benzyl ester | ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C16H18N4O2/c17-15(18)20-14-8-6-12(7-9-14)10-19-16(21)22-11-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,19,21)(H4,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228422

((4-guanidino-benzyl)-carbamic acid benzyl ester | ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C16H18N4O2/c17-15(18)20-14-8-6-12(7-9-14)10-19-16(21)22-11-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,19,21)(H4,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228422

((4-guanidino-benzyl)-carbamic acid benzyl ester | ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C16H18N4O2/c17-15(18)20-14-8-6-12(7-9-14)10-19-16(21)22-11-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,19,21)(H4,17,18,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228422

((4-guanidino-benzyl)-carbamic acid benzyl ester | ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C16H18N4O2/c17-15(18)20-14-8-6-12(7-9-14)10-19-16(21)22-11-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,19,21)(H4,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228418
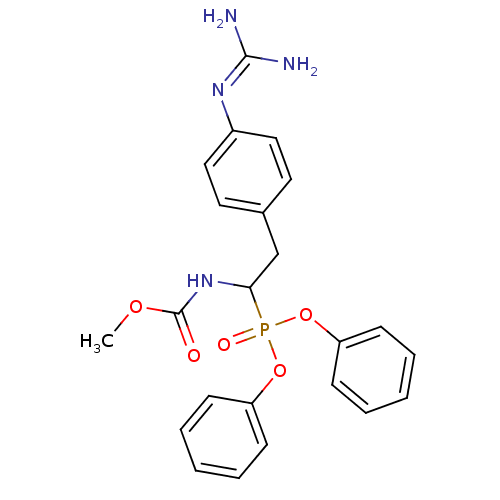
(CHEMBL393591 | methyl 1-(diphenoxyphosphoryl)-2-(4...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C23H25N4O5P/c1-30-23(28)27-21(16-17-12-14-18(15-13-17)26-22(24)25)33(29,31-19-8-4-2-5-9-19)32-20-10-6-3-7-11-20/h2-15,21H,16H2,1H3,(H,27,28)(H4,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228412

(CHEMBL393979 | methyl 1-(bis(4-acetamidophenoxy)ph...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1)[#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1 Show InChI InChI=1S/C27H31N6O7P/c1-17(34)30-20-8-12-23(13-9-20)39-41(37,40-24-14-10-21(11-15-24)31-18(2)35)25(33-27(36)38-3)16-19-4-6-22(7-5-19)32-26(28)29/h4-15,25H,16H2,1-3H3,(H,30,34)(H,31,35)(H,33,36)(H4,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228425

(CHEMBL239118 | di-(4-acetamidophenyl) 1-(methylsul...)Show SMILES [#6]-[#6](=O)-[#7]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#7]-[#6](-[#6])=O)cc2)[#6](-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])-[#7]S([#6])(=O)=O)cc1 Show InChI InChI=1S/C26H31N6O7PS/c1-17(33)29-20-8-12-23(13-9-20)38-40(35,39-24-14-10-21(11-15-24)30-18(2)34)25(32-41(3,36)37)16-19-4-6-22(7-5-19)31-26(27)28/h4-15,25,32H,16H2,1-3H3,(H,29,33)(H,30,34)(H4,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50194743
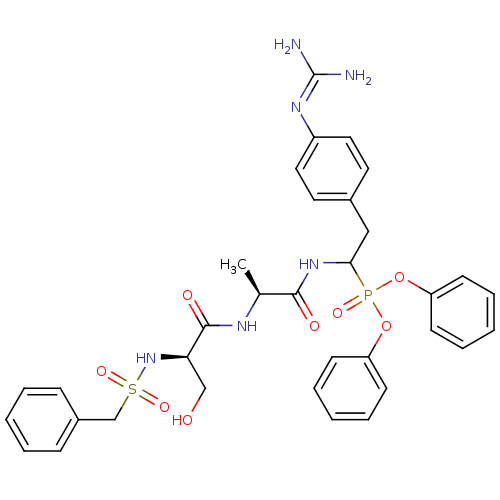
(CHEMBL214814 | diphenyl 1-[(N-alpha-toluenesulfony...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C34H39N6O8PS/c1-24(37-33(43)30(22-41)40-50(45,46)23-26-11-5-2-6-12-26)32(42)39-31(21-25-17-19-27(20-18-25)38-34(35)36)49(44,47-28-13-7-3-8-14-28)48-29-15-9-4-10-16-29/h2-20,24,30-31,40-41H,21-23H2,1H3,(H,37,43)(H,39,42)(H4,35,36,38)/t24-,30+,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228420
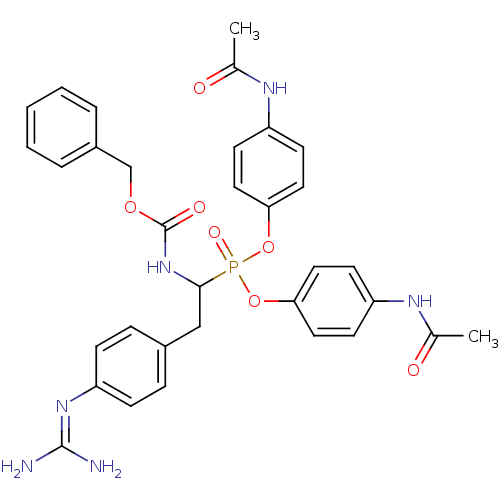
(CHEMBL391968 | di-(4-acetamidophenyl) 1-(benzyloxy...)Show SMILES [#6]-[#6](=O)-[#7]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#7]-[#6](-[#6])=O)cc2)[#6](-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C33H35N6O7P/c1-22(40)36-26-12-16-29(17-13-26)45-47(43,46-30-18-14-27(15-19-30)37-23(2)41)31(20-24-8-10-28(11-9-24)38-32(34)35)39-33(42)44-21-25-6-4-3-5-7-25/h3-19,31H,20-21H2,1-2H3,(H,36,40)(H,37,41)(H,39,42)(H4,34,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228421
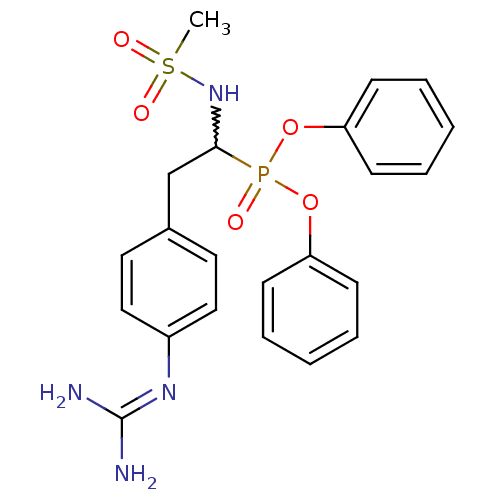
(CHEMBL238493 | diphenyl 1-(methylsulfonylamino)-2-...)Show SMILES [#6]S(=O)(=O)[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 |w:5.4| Show InChI InChI=1S/C22H25N4O5PS/c1-33(28,29)26-21(16-17-12-14-18(15-13-17)25-22(23)24)32(27,30-19-8-4-2-5-9-19)31-20-10-6-3-7-11-20/h2-15,21,26H,16H2,1H3,(H4,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228410
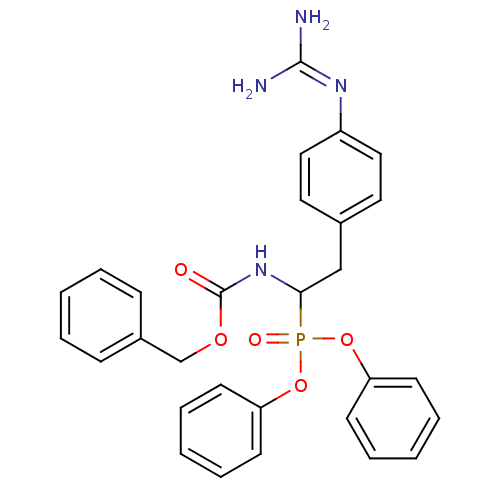
(CHEMBL239535 | diphenyl 1-(benzyloxycarbonylamino)...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#6](-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)P(=O)([#8]-c2ccccc2)[#8]-c2ccccc2)cc1 Show InChI InChI=1S/C29H29N4O5P/c30-28(31)32-24-18-16-22(17-19-24)20-27(33-29(34)36-21-23-10-4-1-5-11-23)39(35,37-25-12-6-2-7-13-25)38-26-14-8-3-9-15-26/h1-19,27H,20-21H2,(H,33,34)(H4,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228414
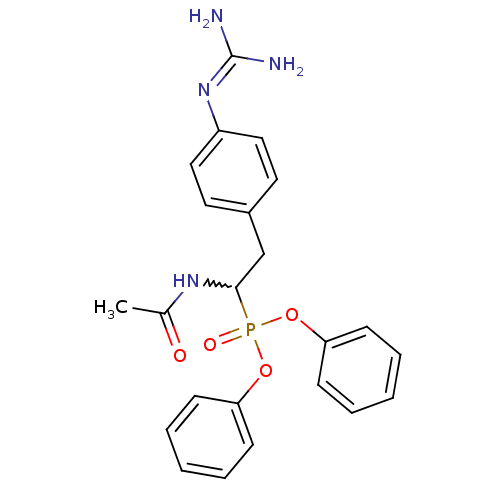
(CHEMBL239747 | diphenyl 1-acetamido-2-(4-guanidino...)Show SMILES [#6]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 |w:4.3| Show InChI InChI=1S/C23H25N4O4P/c1-17(28)26-22(16-18-12-14-19(15-13-18)27-23(24)25)32(29,30-20-8-4-2-5-9-20)31-21-10-6-3-7-11-21/h2-15,22H,16H2,1H3,(H,26,28)(H4,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228417
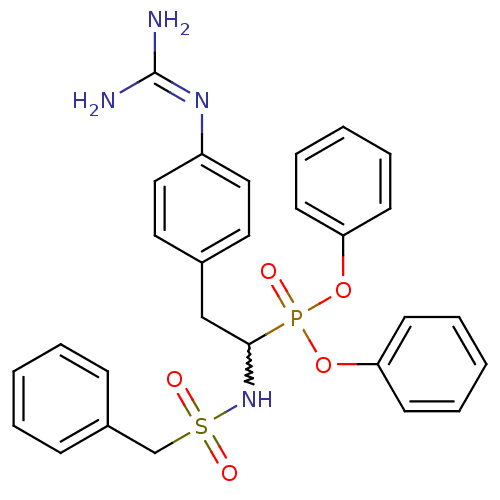
(CHEMBL238491 | diphenyl 1-(o-toluenesulfonylamino)...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#6](-[#7]S(=O)(=O)[#6]-c2ccccc2)P(=O)([#8]-c2ccccc2)[#8]-c2ccccc2)cc1 |w:9.9| Show InChI InChI=1S/C28H29N4O5PS/c29-28(30)31-24-18-16-22(17-19-24)20-27(32-39(34,35)21-23-10-4-1-5-11-23)38(33,36-25-12-6-2-7-13-25)37-26-14-8-3-9-15-26/h1-19,27,32H,20-21H2,(H4,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50228412

(CHEMBL393979 | methyl 1-(bis(4-acetamidophenoxy)ph...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1)[#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1 Show InChI InChI=1S/C27H31N6O7P/c1-17(34)30-20-8-12-23(13-9-20)39-41(37,40-24-14-10-21(11-15-24)31-18(2)35)25(33-27(36)38-3)16-19-4-6-22(7-5-19)32-26(28)29/h4-15,25H,16H2,1-3H3,(H,30,34)(H,31,35)(H,33,36)(H4,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228423
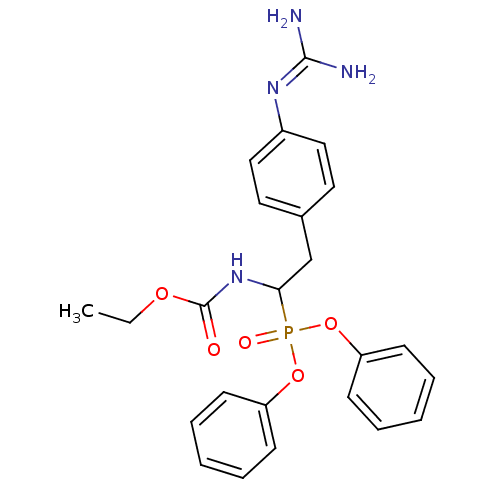
(CHEMBL239536 | ethyl 1-(diphenoxyphosphoryl)-2-(4-...)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C24H27N4O5P/c1-2-31-24(29)28-22(17-18-13-15-19(16-14-18)27-23(25)26)34(30,32-20-9-5-3-6-10-20)33-21-11-7-4-8-12-21/h3-16,22H,2,17H2,1H3,(H,28,29)(H4,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Rattus norvegicus) | BDBM50228418

(CHEMBL393591 | methyl 1-(diphenoxyphosphoryl)-2-(4...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C23H25N4O5P/c1-30-23(28)27-21(16-17-12-14-18(15-13-17)26-22(24)25)33(29,31-19-8-4-2-5-9-19)32-20-10-6-3-7-11-20/h2-15,21H,16H2,1H3,(H,27,28)(H4,24,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of rat uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Rattus norvegicus) | BDBM50228412

(CHEMBL393979 | methyl 1-(bis(4-acetamidophenoxy)ph...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1)[#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1 Show InChI InChI=1S/C27H31N6O7P/c1-17(34)30-20-8-12-23(13-9-20)39-41(37,40-24-14-10-21(11-15-24)31-18(2)35)25(33-27(36)38-3)16-19-4-6-22(7-5-19)32-26(28)29/h4-15,25H,16H2,1-3H3,(H,30,34)(H,31,35)(H,33,36)(H4,28,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of rat uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228426
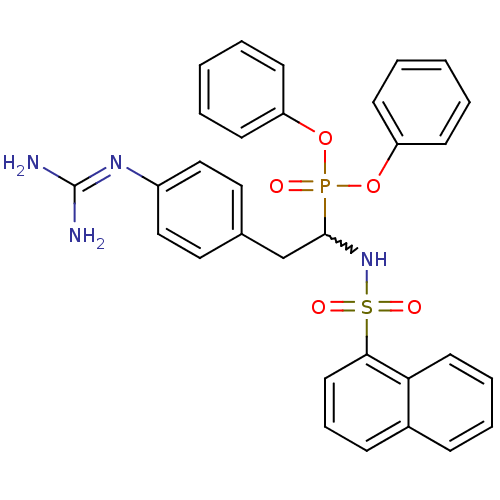
(CHEMBL391344 | diphenyl 1-(naphthalenesulfonylamin...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#6](-[#7]S(=O)(=O)c2cccc3ccccc23)P(=O)([#8]-c2ccccc2)[#8]-c2ccccc2)cc1 |w:9.9| Show InChI InChI=1S/C31H29N4O5PS/c32-31(33)34-25-20-18-23(19-21-25)22-30(35-42(37,38)29-17-9-11-24-10-7-8-16-28(24)29)41(36,39-26-12-3-1-4-13-26)40-27-14-5-2-6-15-27/h1-21,30,35H,22H2,(H4,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50228418

(CHEMBL393591 | methyl 1-(diphenoxyphosphoryl)-2-(4...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C23H25N4O5P/c1-30-23(28)27-21(16-17-12-14-18(15-13-17)26-22(24)25)33(29,31-19-8-4-2-5-9-19)32-20-10-6-3-7-11-20/h2-15,21H,16H2,1H3,(H,27,28)(H4,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50194743

(CHEMBL214814 | diphenyl 1-[(N-alpha-toluenesulfony...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C34H39N6O8PS/c1-24(37-33(43)30(22-41)40-50(45,46)23-26-11-5-2-6-12-26)32(42)39-31(21-25-17-19-27(20-18-25)38-34(35)36)49(44,47-28-13-7-3-8-14-28)48-29-15-9-4-10-16-29/h2-20,24,30-31,40-41H,21-23H2,1H3,(H,37,43)(H,39,42)(H4,35,36,38)/t24-,30+,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228413

(CHEMBL238708 | [1-Benzyloxycarbonylamino-2-(4-carb...)Show SMILES NC(=N)c1ccc(CC(NC(=O)OCc2ccccc2)P(=O)(Oc2ccccc2)Oc2ccccc2)cc1 Show InChI InChI=1S/C29H28N3O5P/c30-28(31)24-18-16-22(17-19-24)20-27(32-29(33)35-21-23-10-4-1-5-11-23)38(34,36-25-12-6-2-7-13-25)37-26-14-8-3-9-15-26/h1-19,27H,20-21H2,(H3,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228424
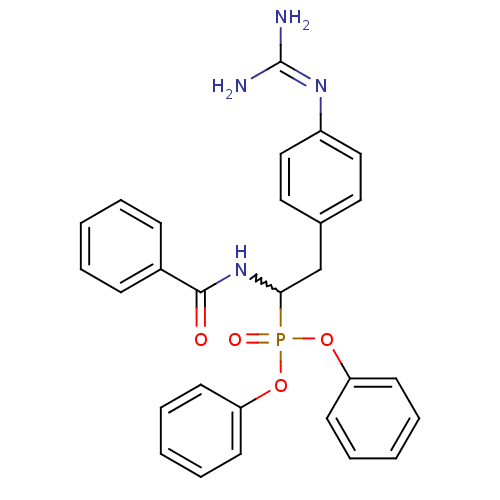
(CHEMBL393592 | diphenyl 1-(benzoylamino)-2-(4-guan...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#6](-[#7]-[#6](=O)-c2ccccc2)P(=O)([#8]-c2ccccc2)[#8]-c2ccccc2)cc1 |w:9.9| Show InChI InChI=1S/C28H27N4O4P/c29-28(30)31-23-18-16-21(17-19-23)20-26(32-27(33)22-10-4-1-5-11-22)37(34,35-24-12-6-2-7-13-24)36-25-14-8-3-9-15-25/h1-19,26H,20H2,(H,32,33)(H4,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228411
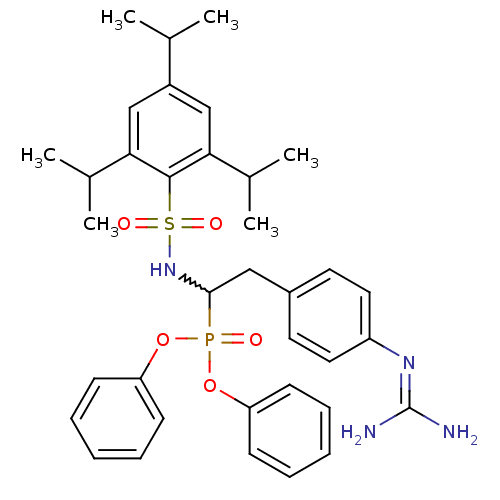
(CHEMBL391916 | diphenyl 1-(2,3,6-tri-isopropylbenz...)Show SMILES [#6]-[#6](-[#6])-c1cc(-[#6](-[#6])-[#6])c(c(c1)-[#6](-[#6])-[#6])S(=O)(=O)[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 |w:19.19| Show InChI InChI=1S/C36H45N4O5PS/c1-24(2)28-22-32(25(3)4)35(33(23-28)26(5)6)47(42,43)40-34(21-27-17-19-29(20-18-27)39-36(37)38)46(41,44-30-13-9-7-10-14-30)45-31-15-11-8-12-16-31/h7-20,22-26,34,40H,21H2,1-6H3,(H4,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50194743

(CHEMBL214814 | diphenyl 1-[(N-alpha-toluenesulfony...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C34H39N6O8PS/c1-24(37-33(43)30(22-41)40-50(45,46)23-26-11-5-2-6-12-26)32(42)39-31(21-25-17-19-27(20-18-25)38-34(35)36)49(44,47-28-13-7-3-8-14-28)48-29-15-9-4-10-16-29/h2-20,24,30-31,40-41H,21-23H2,1H3,(H,37,43)(H,39,42)(H4,35,36,38)/t24-,30+,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228415
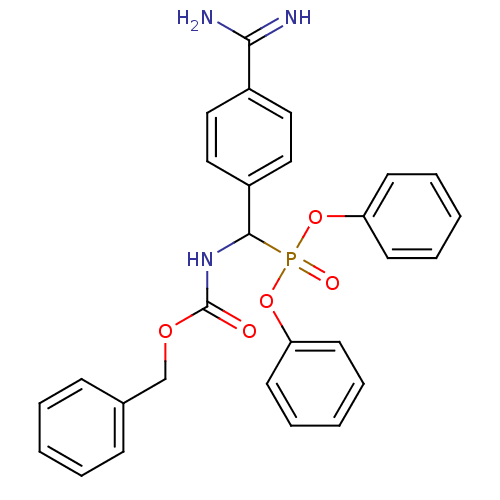
(CHEMBL238707 | [Benzyloxycarbonylamino-(4-carbamim...)Show SMILES NC(=N)c1ccc(cc1)C(NC(=O)OCc1ccccc1)P(=O)(Oc1ccccc1)Oc1ccccc1 Show InChI InChI=1S/C28H26N3O5P/c29-26(30)22-16-18-23(19-17-22)27(31-28(32)34-20-21-10-4-1-5-11-21)37(33,35-24-12-6-2-7-13-24)36-25-14-8-3-9-15-25/h1-19,27H,20H2,(H3,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228420

(CHEMBL391968 | di-(4-acetamidophenyl) 1-(benzyloxy...)Show SMILES [#6]-[#6](=O)-[#7]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#7]-[#6](-[#6])=O)cc2)[#6](-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C33H35N6O7P/c1-22(40)36-26-12-16-29(17-13-26)45-47(43,46-30-18-14-27(15-19-30)37-23(2)41)31(20-24-8-10-28(11-9-24)38-32(34)35)39-33(42)44-21-25-6-4-3-5-7-25/h3-19,31H,20-21H2,1-2H3,(H,36,40)(H,37,41)(H,39,42)(H4,34,35,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50194743

(CHEMBL214814 | diphenyl 1-[(N-alpha-toluenesulfony...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C34H39N6O8PS/c1-24(37-33(43)30(22-41)40-50(45,46)23-26-11-5-2-6-12-26)32(42)39-31(21-25-17-19-27(20-18-25)38-34(35)36)49(44,47-28-13-7-3-8-14-28)48-29-15-9-4-10-16-29/h2-20,24,30-31,40-41H,21-23H2,1H3,(H,37,43)(H,39,42)(H4,35,36,38)/t24-,30+,31?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228416

(CHEMBL239331 | diphenyl N-(benzyloxycarbonylamino)...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#8]-[#6]-c1ccccc1)P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C25H29N4O5P/c26-24(27)28-18-10-17-23(29-25(30)32-19-20-11-4-1-5-12-20)35(31,33-21-13-6-2-7-14-21)34-22-15-8-3-9-16-22/h1-9,11-16,23H,10,17-19H2,(H,29,30)(H4,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228416

(CHEMBL239331 | diphenyl N-(benzyloxycarbonylamino)...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#8]-[#6]-c1ccccc1)P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C25H29N4O5P/c26-24(27)28-18-10-17-23(29-25(30)32-19-20-11-4-1-5-12-20)35(31,33-21-13-6-2-7-14-21)34-22-15-8-3-9-16-22/h1-9,11-16,23H,10,17-19H2,(H,29,30)(H4,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228420

(CHEMBL391968 | di-(4-acetamidophenyl) 1-(benzyloxy...)Show SMILES [#6]-[#6](=O)-[#7]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#7]-[#6](-[#6])=O)cc2)[#6](-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C33H35N6O7P/c1-22(40)36-26-12-16-29(17-13-26)45-47(43,46-30-18-14-27(15-19-30)37-23(2)41)31(20-24-8-10-28(11-9-24)38-32(34)35)39-33(42)44-21-25-6-4-3-5-7-25/h3-19,31H,20-21H2,1-2H3,(H,36,40)(H,37,41)(H,39,42)(H4,34,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228419
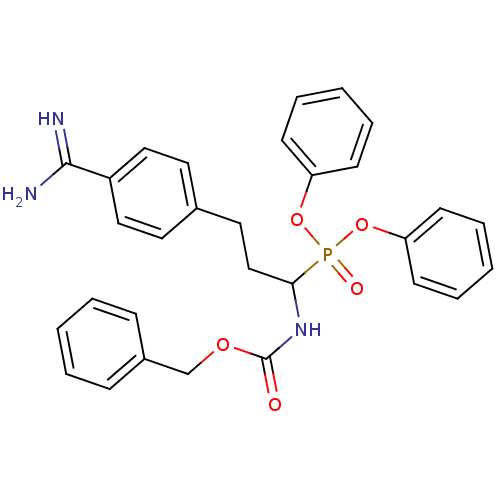
(CHEMBL238913 | diphenyl 1-(N-benzyloxycarbonylamin...)Show SMILES NC(=N)c1ccc(CCC(NC(=O)OCc2ccccc2)P(=O)(Oc2ccccc2)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H30N3O5P/c31-29(32)25-19-16-23(17-20-25)18-21-28(33-30(34)36-22-24-10-4-1-5-11-24)39(35,37-26-12-6-2-7-13-26)38-27-14-8-3-9-15-27/h1-17,19-20,28H,18,21-22H2,(H3,31,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228412

(CHEMBL393979 | methyl 1-(bis(4-acetamidophenoxy)ph...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1)[#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1 Show InChI InChI=1S/C27H31N6O7P/c1-17(34)30-20-8-12-23(13-9-20)39-41(37,40-24-14-10-21(11-15-24)31-18(2)35)25(33-27(36)38-3)16-19-4-6-22(7-5-19)32-26(28)29/h4-15,25H,16H2,1-3H3,(H,30,34)(H,31,35)(H,33,36)(H4,28,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228413

(CHEMBL238708 | [1-Benzyloxycarbonylamino-2-(4-carb...)Show SMILES NC(=N)c1ccc(CC(NC(=O)OCc2ccccc2)P(=O)(Oc2ccccc2)Oc2ccccc2)cc1 Show InChI InChI=1S/C29H28N3O5P/c30-28(31)24-18-16-22(17-19-24)20-27(32-29(33)35-21-23-10-4-1-5-11-23)38(34,36-25-12-6-2-7-13-25)37-26-14-8-3-9-15-26/h1-19,27H,20-21H2,(H3,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228426

(CHEMBL391344 | diphenyl 1-(naphthalenesulfonylamin...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#6](-[#7]S(=O)(=O)c2cccc3ccccc23)P(=O)([#8]-c2ccccc2)[#8]-c2ccccc2)cc1 |w:9.9| Show InChI InChI=1S/C31H29N4O5PS/c32-31(33)34-25-20-18-23(19-21-25)22-30(35-42(37,38)29-17-9-11-24-10-7-8-16-28(24)29)41(36,39-26-12-3-1-4-13-26)40-27-14-5-2-6-15-27/h1-21,30,35H,22H2,(H4,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228428

(CHEMBL238706 | diphenyl 1-amino-2-(4-guanidinophen...)Show SMILES [#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C21H23N4O3P/c22-20(15-16-11-13-17(14-12-16)25-21(23)24)29(26,27-18-7-3-1-4-8-18)28-19-9-5-2-6-10-19/h1-14,20H,15,22H2,(H4,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228425

(CHEMBL239118 | di-(4-acetamidophenyl) 1-(methylsul...)Show SMILES [#6]-[#6](=O)-[#7]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#7]-[#6](-[#6])=O)cc2)[#6](-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])-[#7]S([#6])(=O)=O)cc1 Show InChI InChI=1S/C26H31N6O7PS/c1-17(33)29-20-8-12-23(13-9-20)38-40(35,39-24-14-10-21(11-15-24)30-18(2)34)25(32-41(3,36)37)16-19-4-6-22(7-5-19)31-26(27)28/h4-15,25,32H,16H2,1-3H3,(H,29,33)(H,30,34)(H4,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Rattus norvegicus) | BDBM50228412

(CHEMBL393979 | methyl 1-(bis(4-acetamidophenoxy)ph...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1)[#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1 Show InChI InChI=1S/C27H31N6O7P/c1-17(34)30-20-8-12-23(13-9-20)39-41(37,40-24-14-10-21(11-15-24)31-18(2)35)25(33-27(36)38-3)16-19-4-6-22(7-5-19)32-26(28)29/h4-15,25H,16H2,1-3H3,(H,30,34)(H,31,35)(H,33,36)(H4,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of rat plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228425

(CHEMBL239118 | di-(4-acetamidophenyl) 1-(methylsul...)Show SMILES [#6]-[#6](=O)-[#7]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#7]-[#6](-[#6])=O)cc2)[#6](-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])-[#7]S([#6])(=O)=O)cc1 Show InChI InChI=1S/C26H31N6O7PS/c1-17(33)29-20-8-12-23(13-9-20)38-40(35,39-24-14-10-21(11-15-24)30-18(2)34)25(32-41(3,36)37)16-19-4-6-22(7-5-19)31-26(27)28/h4-15,25,32H,16H2,1-3H3,(H,29,33)(H,30,34)(H4,27,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228410

(CHEMBL239535 | diphenyl 1-(benzyloxycarbonylamino)...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#6](-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)P(=O)([#8]-c2ccccc2)[#8]-c2ccccc2)cc1 Show InChI InChI=1S/C29H29N4O5P/c30-28(31)32-24-18-16-22(17-19-24)20-27(33-29(34)36-21-23-10-4-1-5-11-23)39(35,37-25-12-6-2-7-13-25)38-26-14-8-3-9-15-26/h1-19,27H,20-21H2,(H,33,34)(H4,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228412

(CHEMBL393979 | methyl 1-(bis(4-acetamidophenoxy)ph...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1)[#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1 Show InChI InChI=1S/C27H31N6O7P/c1-17(34)30-20-8-12-23(13-9-20)39-41(37,40-24-14-10-21(11-15-24)31-18(2)35)25(33-27(36)38-3)16-19-4-6-22(7-5-19)32-26(28)29/h4-15,25H,16H2,1-3H3,(H,30,34)(H,31,35)(H,33,36)(H4,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228410

(CHEMBL239535 | diphenyl 1-(benzyloxycarbonylamino)...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#6](-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)P(=O)([#8]-c2ccccc2)[#8]-c2ccccc2)cc1 Show InChI InChI=1S/C29H29N4O5P/c30-28(31)32-24-18-16-22(17-19-24)20-27(33-29(34)36-21-23-10-4-1-5-11-23)39(35,37-25-12-6-2-7-13-25)38-26-14-8-3-9-15-26/h1-19,27H,20-21H2,(H,33,34)(H4,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228415

(CHEMBL238707 | [Benzyloxycarbonylamino-(4-carbamim...)Show SMILES NC(=N)c1ccc(cc1)C(NC(=O)OCc1ccccc1)P(=O)(Oc1ccccc1)Oc1ccccc1 Show InChI InChI=1S/C28H26N3O5P/c29-26(30)22-16-18-23(19-17-22)27(31-28(32)34-20-21-10-4-1-5-11-21)37(33,35-24-12-6-2-7-13-24)36-25-14-8-3-9-15-25/h1-19,27H,20H2,(H3,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228425

(CHEMBL239118 | di-(4-acetamidophenyl) 1-(methylsul...)Show SMILES [#6]-[#6](=O)-[#7]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#7]-[#6](-[#6])=O)cc2)[#6](-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])-[#7]S([#6])(=O)=O)cc1 Show InChI InChI=1S/C26H31N6O7PS/c1-17(33)29-20-8-12-23(13-9-20)38-40(35,39-24-14-10-21(11-15-24)30-18(2)34)25(32-41(3,36)37)16-19-4-6-22(7-5-19)31-26(27)28/h4-15,25,32H,16H2,1-3H3,(H,29,33)(H,30,34)(H4,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228423

(CHEMBL239536 | ethyl 1-(diphenoxyphosphoryl)-2-(4-...)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C24H27N4O5P/c1-2-31-24(29)28-22(17-18-13-15-19(16-14-18)27-23(25)26)34(30,32-20-9-5-3-6-10-20)33-21-11-7-4-8-12-21/h3-16,22H,2,17H2,1H3,(H,28,29)(H4,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228413

(CHEMBL238708 | [1-Benzyloxycarbonylamino-2-(4-carb...)Show SMILES NC(=N)c1ccc(CC(NC(=O)OCc2ccccc2)P(=O)(Oc2ccccc2)Oc2ccccc2)cc1 Show InChI InChI=1S/C29H28N3O5P/c30-28(31)24-18-16-22(17-19-24)20-27(32-29(33)35-21-23-10-4-1-5-11-23)38(34,36-25-12-6-2-7-13-25)37-26-14-8-3-9-15-26/h1-19,27H,20-21H2,(H3,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Mus musculus) | BDBM50228412

(CHEMBL393979 | methyl 1-(bis(4-acetamidophenoxy)ph...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1)[#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1 Show InChI InChI=1S/C27H31N6O7P/c1-17(34)30-20-8-12-23(13-9-20)39-41(37,40-24-14-10-21(11-15-24)31-18(2)35)25(33-27(36)38-3)16-19-4-6-22(7-5-19)32-26(28)29/h4-15,25H,16H2,1-3H3,(H,30,34)(H,31,35)(H,33,36)(H4,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of mouse thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data