

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Enterobacter cloacae) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class A Beta-lactamase TEM isolated from Enterobacter coli | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092284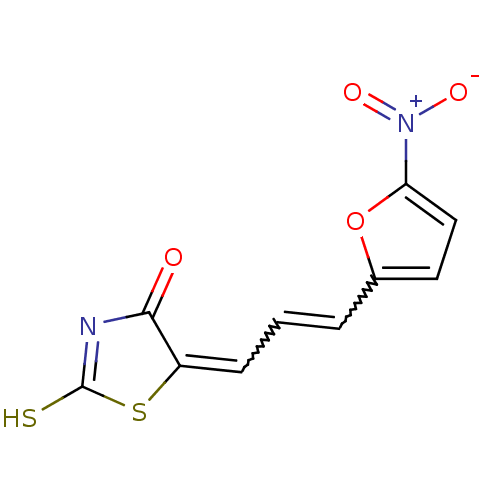 (5-[(E)-3-(5-Nitro-furan-2-yl)-prop-2-en-(Z)-yliden...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class C Beta-lactamase isolated from Enterobacter cloacae | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092281 (3-[4-Oxo-2-thioxo-thiazolidin-(5E)-ylidene]-1,3-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class C Beta-lactamase isolated from Enterobacter cloacae | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471725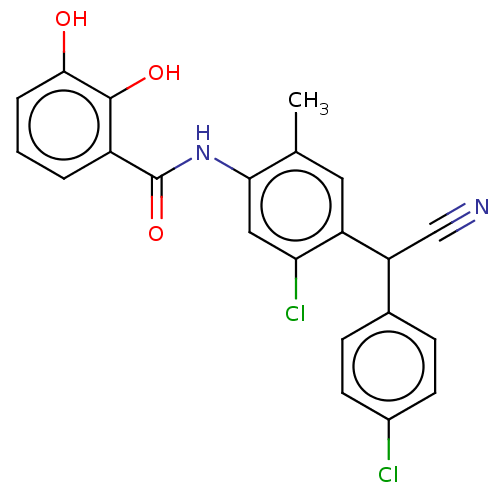 (CHEMBL327734) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215968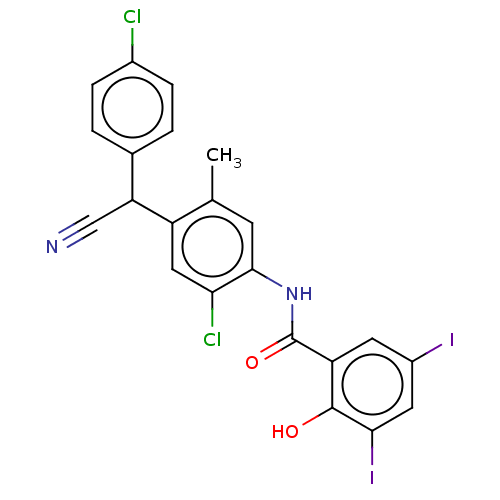 (CHEMBL57324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of histidine protein kinase (KinA) phosphorylation in the presence of response regulator (Spo0F) | Bioorg Med Chem Lett 8: 1923-8 (1998) BindingDB Entry DOI: 10.7270/Q27S7QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50063753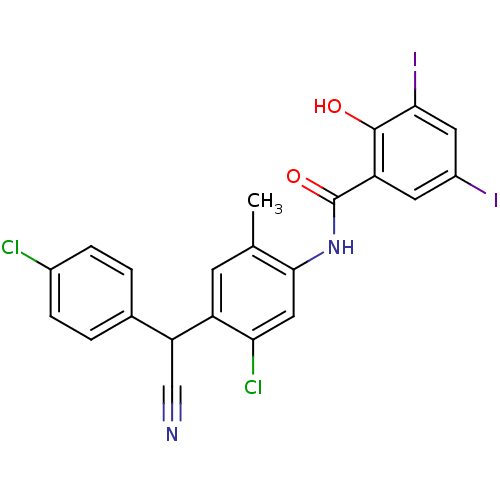 (CHEMBL12131 | Closantel | N-(5-chloro-4-((4-chloro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092283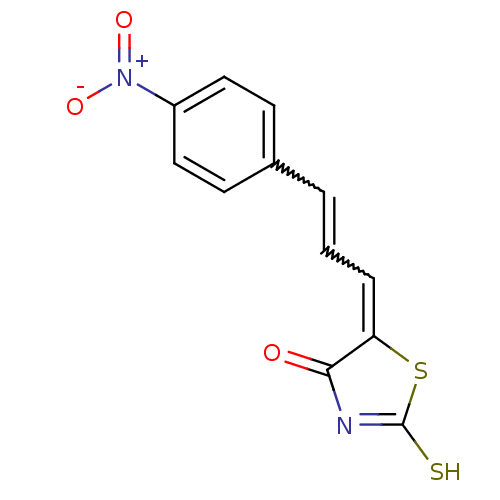 (5-[(E)-3-(4-Nitro-phenyl)-prop-2-en-(Z)-ylidene]-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class C Beta-lactamase isolated from Enterobacter cloacae | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471718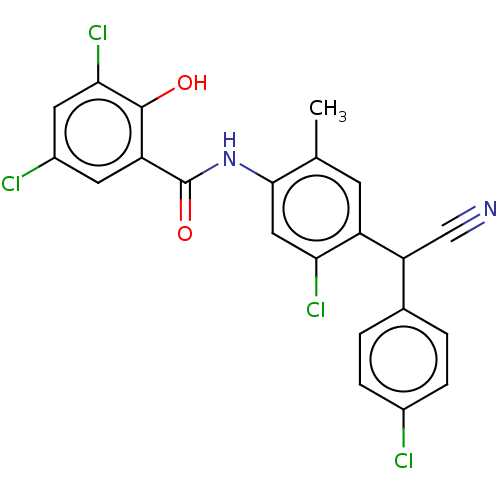 (CHEMBL98026) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215967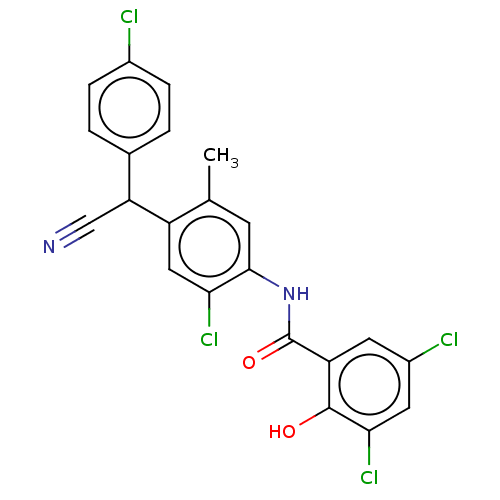 (CHEMBL56579) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of histidine protein kinase (KinA) phosphorylation in the presence of response regulator (Spo0F) | Bioorg Med Chem Lett 8: 1923-8 (1998) BindingDB Entry DOI: 10.7270/Q27S7QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218645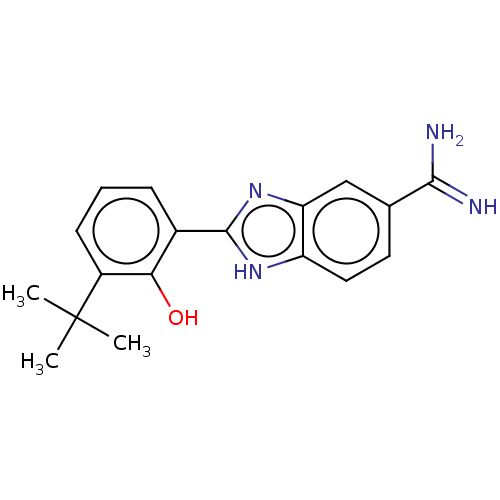 (CHEMBL288462) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218644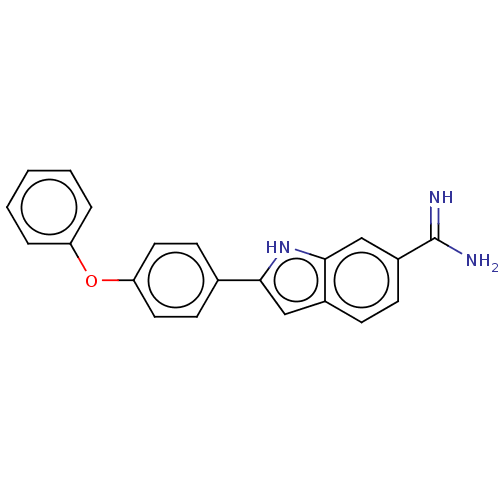 (CHEMBL416972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class C Beta-lactamase isolated from Enterobacter cloacae | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215985 (CHEMBL57037) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit autophosphorylation of KinA/Sp0F assay | Bioorg Med Chem Lett 8: 1929-34 (1998) BindingDB Entry DOI: 10.7270/Q2417070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092286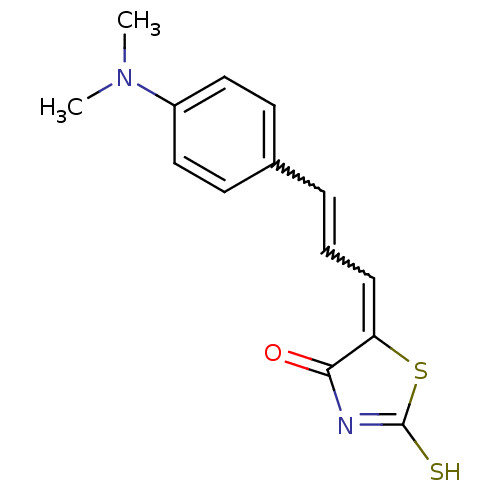 (5-[(E)-3-(4-Dimethylamino-phenyl)-prop-2-en-(Z)-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class C Beta-lactamase isolated from Enterobacter cloacae | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092281 (3-[4-Oxo-2-thioxo-thiazolidin-(5E)-ylidene]-1,3-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class A Beta-lactamase TEM isolated from Enterobacter coli | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471730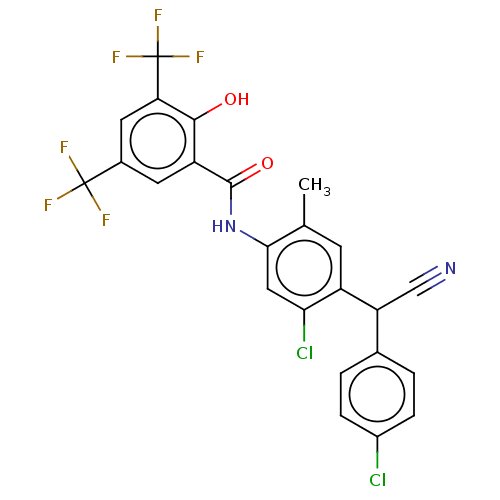 (CHEMBL101786) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation initiation phosphotransferase F/kinase A (Bacillus subtilis (strain 168)) | BDBM50215963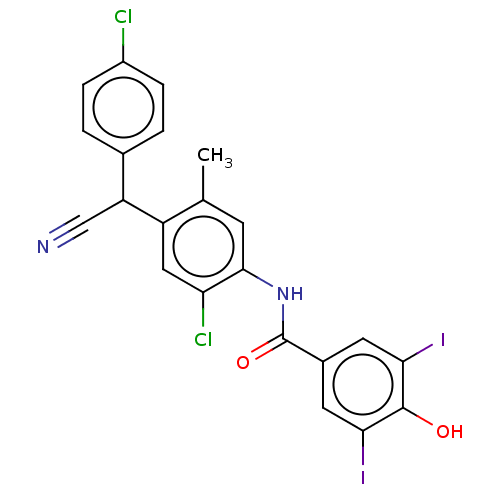 (CHEMBL57687) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of histidine protein kinase (KinA) phosphorylation in the presence of response regulator (Spo0F) | Bioorg Med Chem Lett 8: 1923-8 (1998) BindingDB Entry DOI: 10.7270/Q27S7QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471728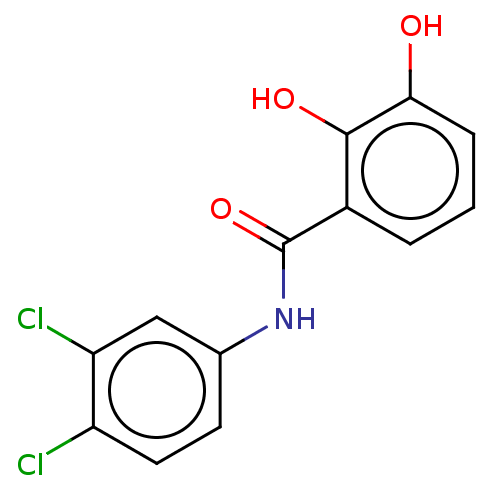 (CHEMBL100719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092279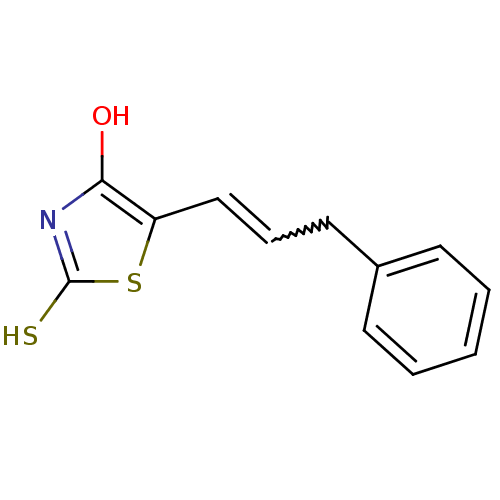 (5-[3-Phenyl-prop-(Z)-ylidene]-2-thioxo-thiazolidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class C Beta-lactamase isolated from Enterobacter cloacae | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218648 (CHEMBL290098) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218640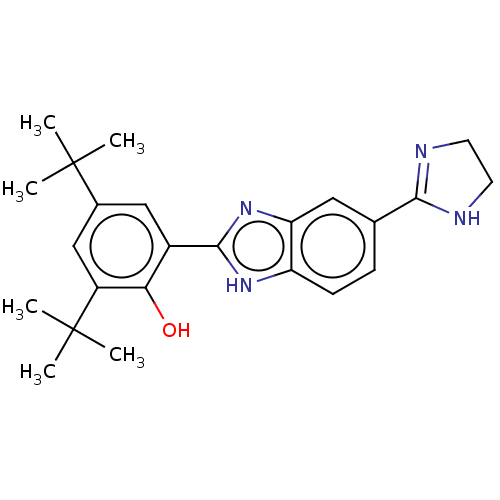 (CHEMBL38683) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218643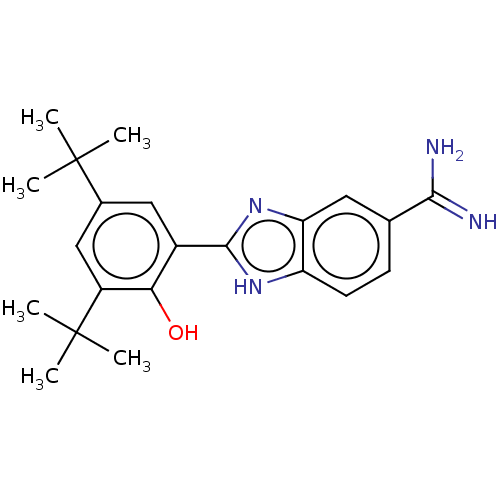 (CHEMBL39634) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218655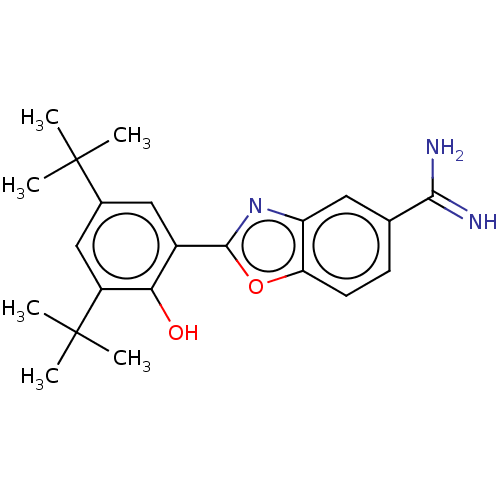 (CHEMBL38914) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218647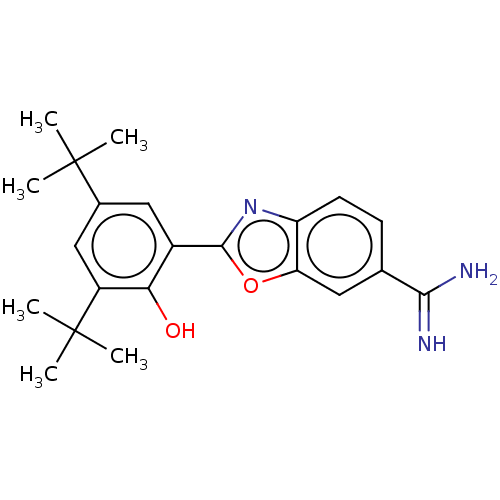 (CHEMBL38867) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215976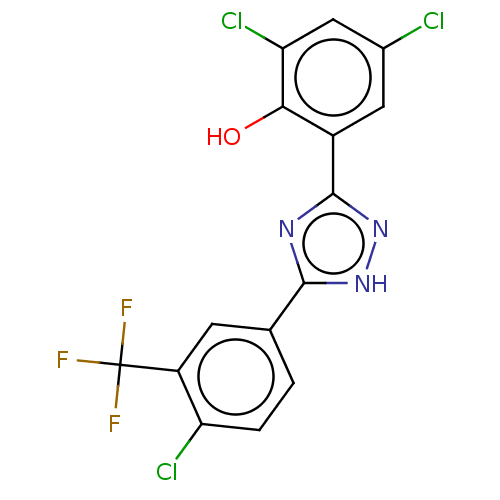 (CHEMBL58536) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit autophosphorylation of KinA/Sp0F assay | Bioorg Med Chem Lett 8: 1929-34 (1998) BindingDB Entry DOI: 10.7270/Q2417070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218659 (CHEMBL43691) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471723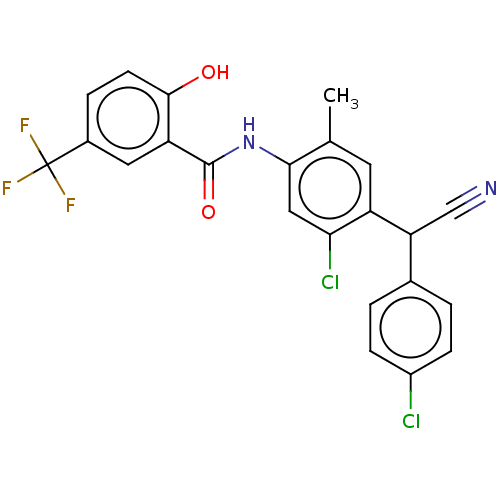 (CHEMBL98273) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50065970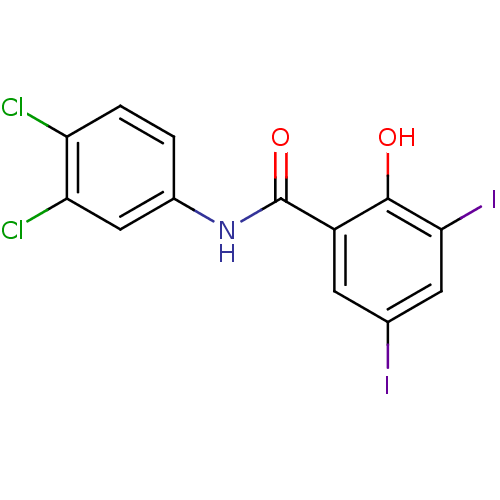 (CHEMBL57656 | N-(3,4-Dichloro-phenyl)-2-hydroxy-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50065970 (CHEMBL57656 | N-(3,4-Dichloro-phenyl)-2-hydroxy-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of histidine protein kinase (KinA) phosphorylation in the presence of response regulator (Spo0F) | Bioorg Med Chem Lett 8: 1923-8 (1998) BindingDB Entry DOI: 10.7270/Q27S7QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215978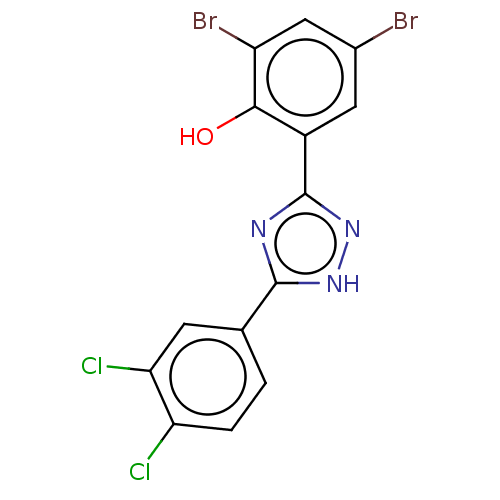 (CHEMBL58439) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit autophosphorylation of KinA/Sp0F assay | Bioorg Med Chem Lett 8: 1929-34 (1998) BindingDB Entry DOI: 10.7270/Q2417070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218653 (CHEMBL39178) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471716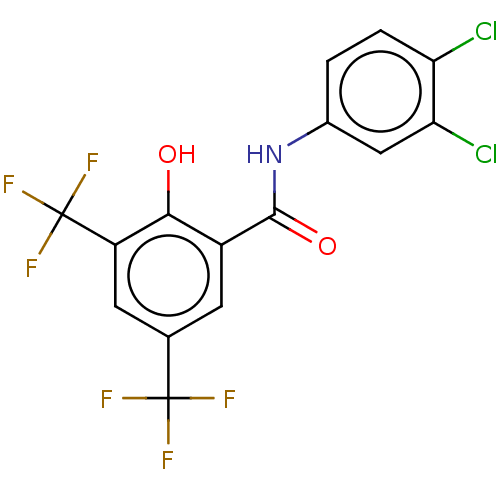 (CHEMBL431272) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50100895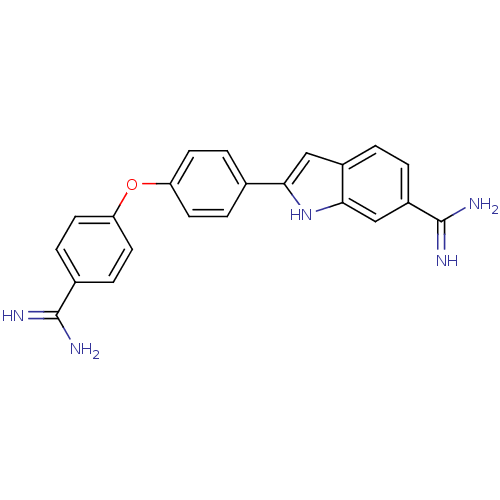 (2-(4-(4-carbamimidoylphenoxy)phenyl)-1H-indole-6-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component system (TCS) from Bacillus subtilis | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092279 (5-[3-Phenyl-prop-(Z)-ylidene]-2-thioxo-thiazolidin...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class A Beta-lactamase TEM isolated from Enterobacter coli | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215974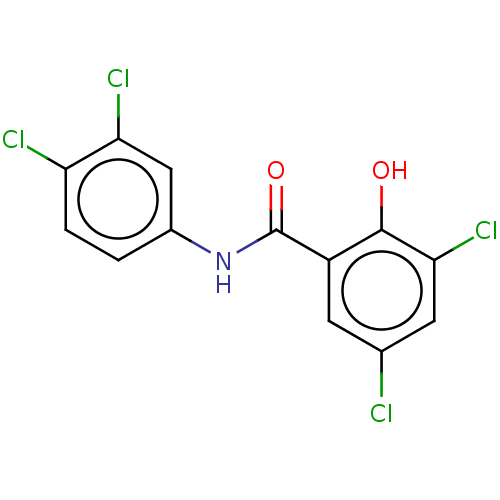 (3,3'',4'',5-tetrachlorosalicylanilide | 3,3',4',5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit autophosphorylation of KinA/Sp0F assay | Bioorg Med Chem Lett 8: 1929-34 (1998) BindingDB Entry DOI: 10.7270/Q2417070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218651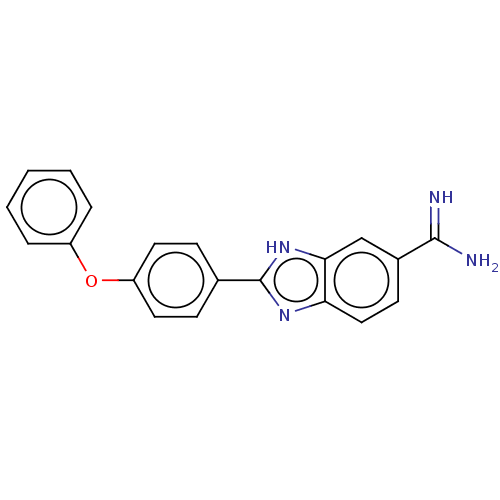 (CHEMBL39490) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F two component system (TCS) from Bacillus subtilis | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092268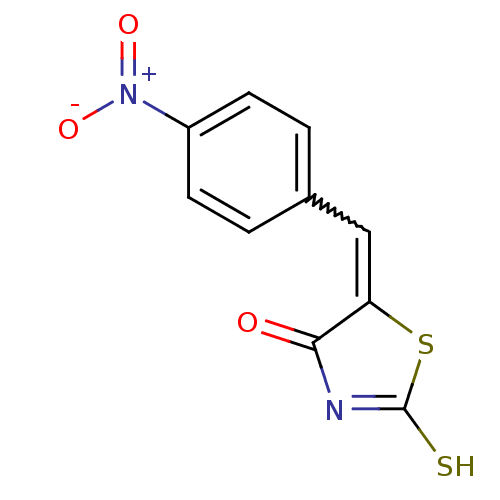 (5-(4-nitrobenzylidene)-2-thioxothiazolidin-4-one |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class C Beta-lactamase isolated from Enterobacter cloacae | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218656 (CHEMBL39040) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215977 (CHEMBL58560) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit autophosphorylation of KinA/Sp0F assay | Bioorg Med Chem Lett 8: 1929-34 (1998) BindingDB Entry DOI: 10.7270/Q2417070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215974 (3,3'',4'',5-tetrachlorosalicylanilide | 3,3',4',5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of histidine protein kinase (KinA) phosphorylation in the presence of response regulator (Spo0F) | Bioorg Med Chem Lett 8: 1923-8 (1998) BindingDB Entry DOI: 10.7270/Q27S7QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215974 (3,3'',4'',5-tetrachlorosalicylanilide | 3,3',4',5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218646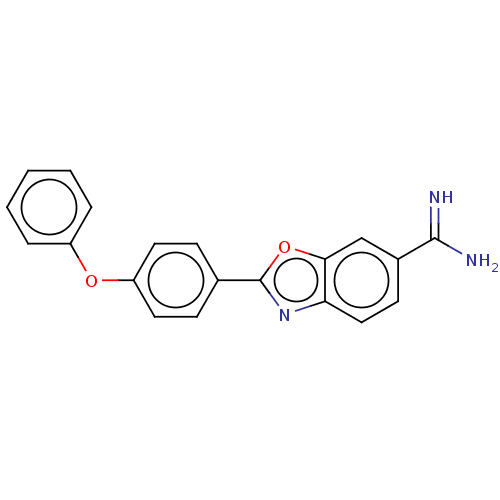 (CHEMBL290549) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092269 (5-[(E)-3-(5-Nitro-furan-2-yl)-prop-2-en-(Z)-yliden...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class A Beta-lactamase TEM isolated from Enterobacter coli | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471712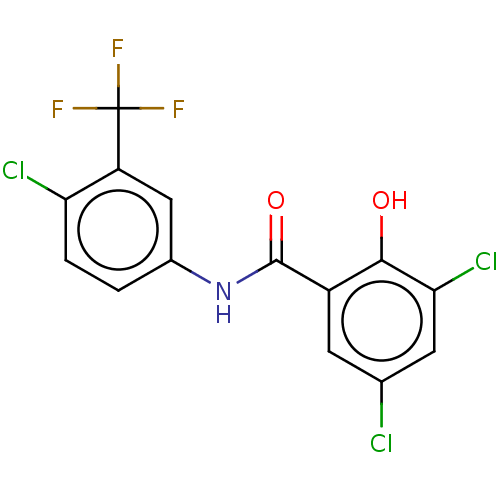 (CHEMBL319252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092269 (5-[(E)-3-(5-Nitro-furan-2-yl)-prop-2-en-(Z)-yliden...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class C Beta-lactamase isolated from Enterobacter cloacae | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50092282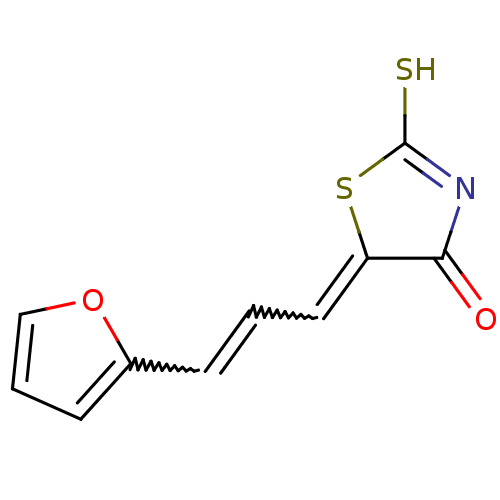 (5-[(E)-3-Furan-2-yl-prop-2-en-(Z)-ylidene]-2-thiox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against class C Beta-lactamase isolated from Enterobacter cloacae | Bioorg Med Chem Lett 10: 2179-82 (2001) BindingDB Entry DOI: 10.7270/Q2T15456 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218654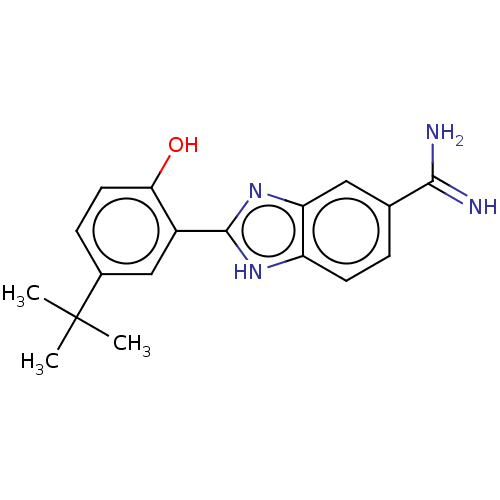 (CHEMBL40096) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50216089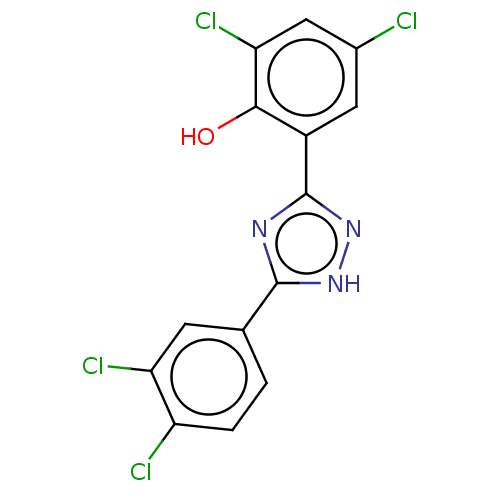 (CHEMBL441791) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit autophosphorylation of KinA/Sp0F assay | Bioorg Med Chem Lett 8: 1929-34 (1998) BindingDB Entry DOI: 10.7270/Q2417070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471719 (CHEMBL100688) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218649 (CHEMBL417892) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 123 total ) | Next | Last >> |