

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581847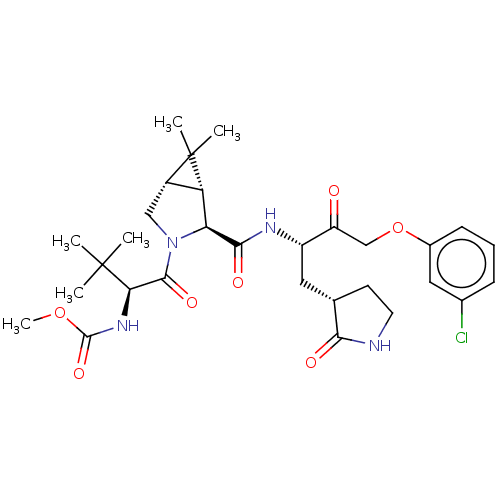 (WO2022208262, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50018850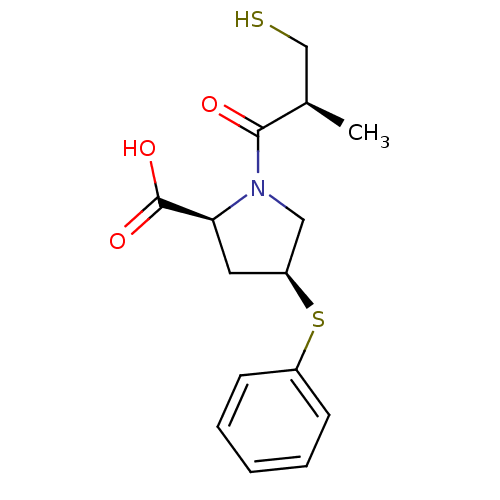 (1-(3-Mercapto-2-methyl-propionyl)-4-phenylsulfanyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory constant against rabbit lung Angiotensin I converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581848 (WO2022208262, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | <0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313984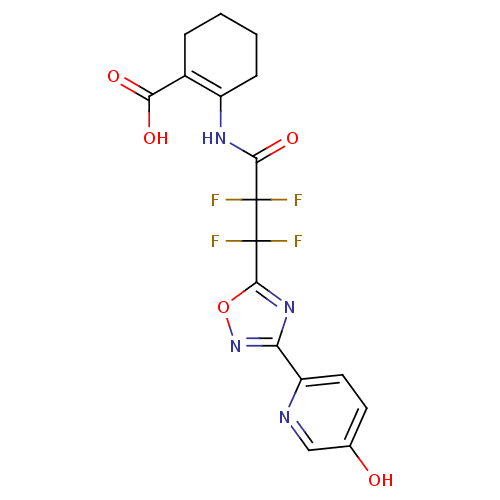 (2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367254 (ENALAPRILAT) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581851 (WO2022208262, Example 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50018849 (4-Cyclohexyl-1-{2-[hydroxy-(4-phenyl-butyl)-phosph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535166 (WO2022013684, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581852 (WO2022208262, Example 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581846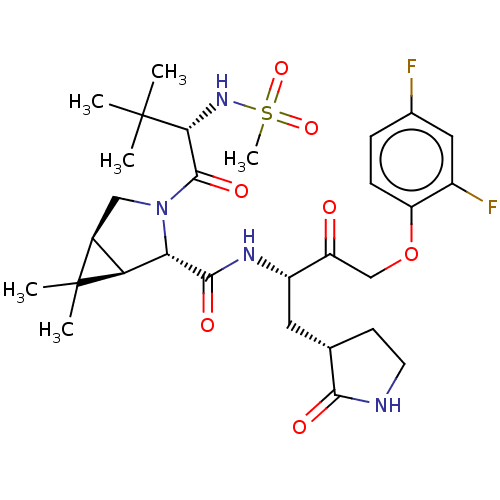 (WO2022208262, Example 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581850 (WO2022208262, Example 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581839 (WO2022208262, Example 1 | WO2022208262, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581855 (WO2022208262, Example 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581857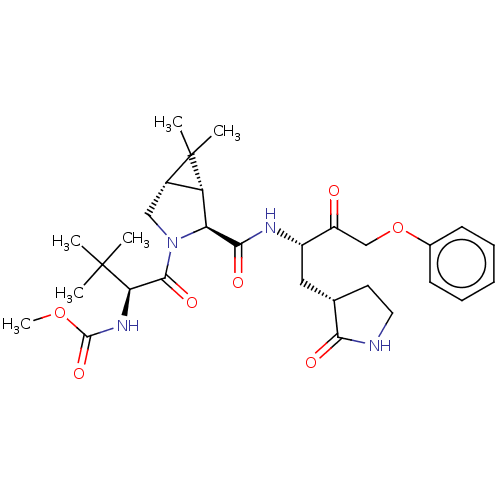 (WO2022208262, Example 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581858 (WO2022208262, Example 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581839 (WO2022208262, Example 1 | WO2022208262, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581860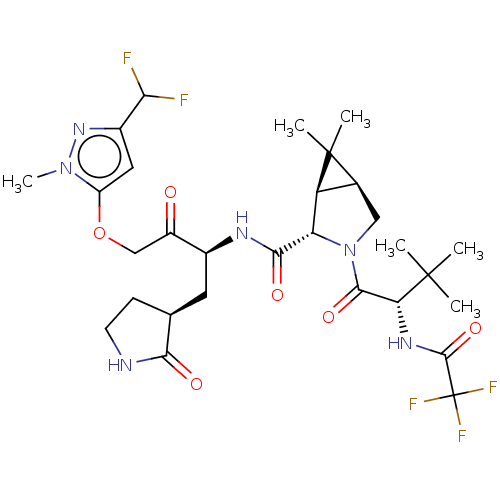 (WO2022208262, Example 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581842 (WO2022208262, Example 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313977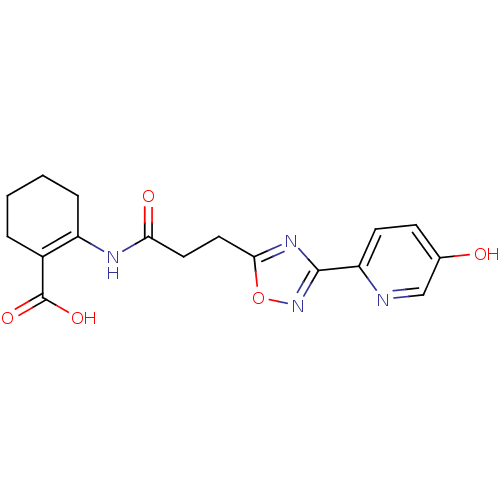 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581859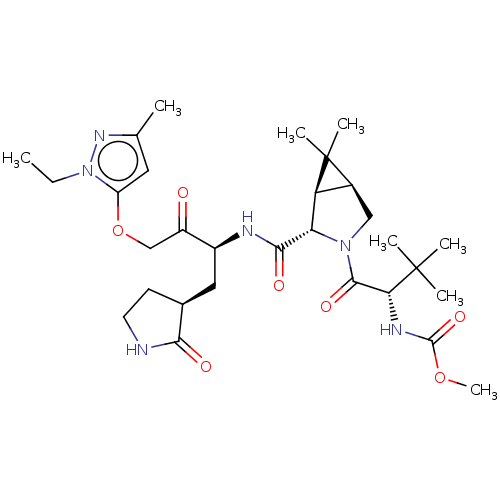 (WO2022208262, Example 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 3.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581840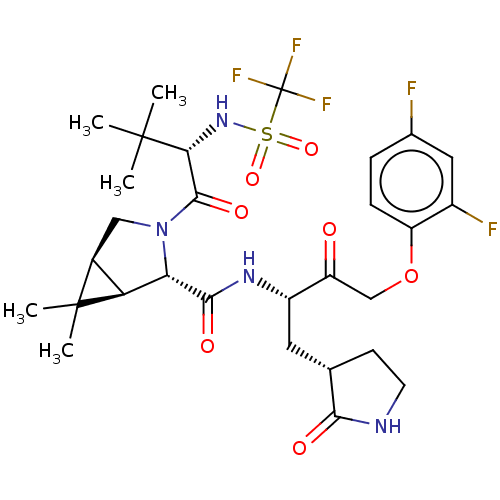 (WO2022208262, Example 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581863 (WO2022208262, Example 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23533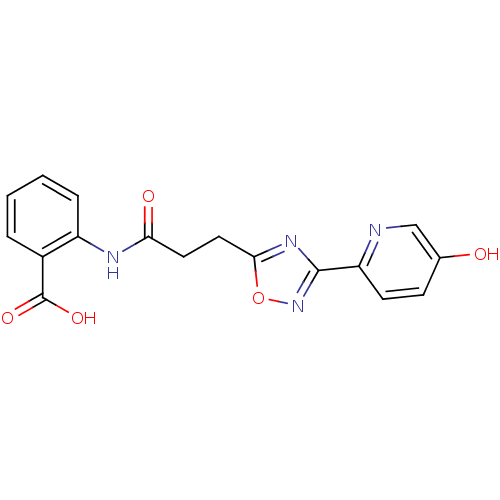 (2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313976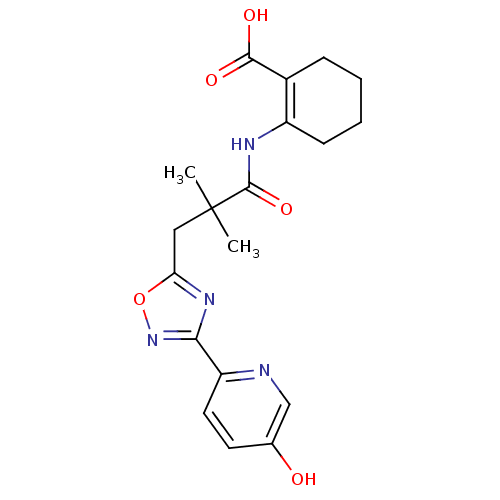 (2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581862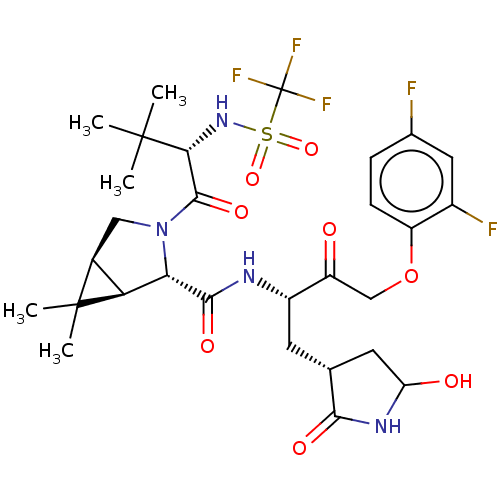 (WO2022208262, Example 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 4.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581864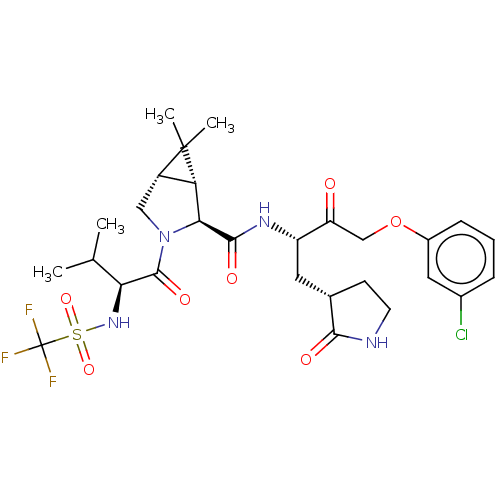 (WO2022208262, Example 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 4.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581865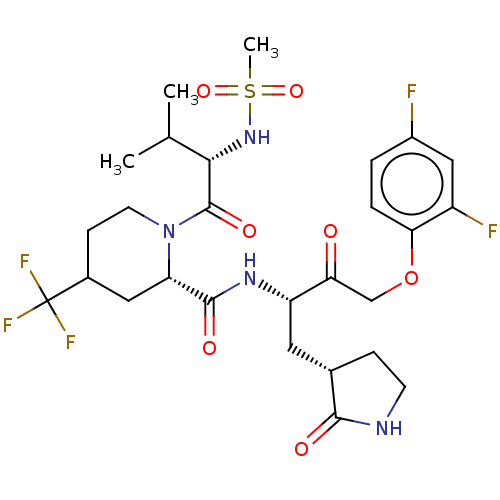 (WO2022208262, Example 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 5.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535159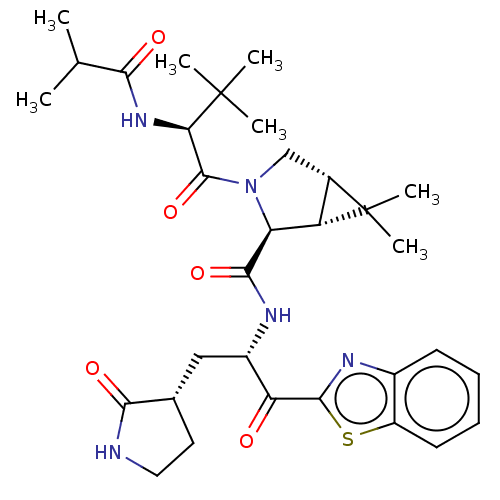 (WO2022013684, Example 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535160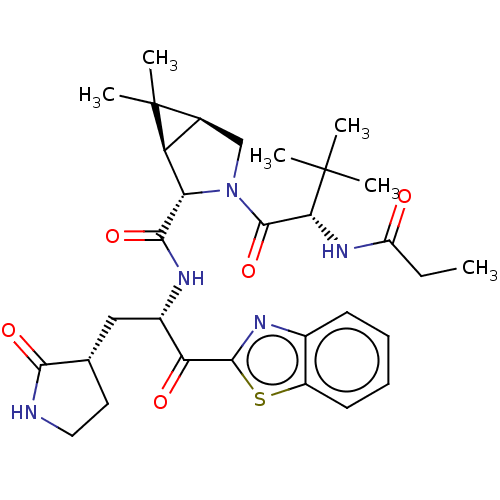 (WO2022013684, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581849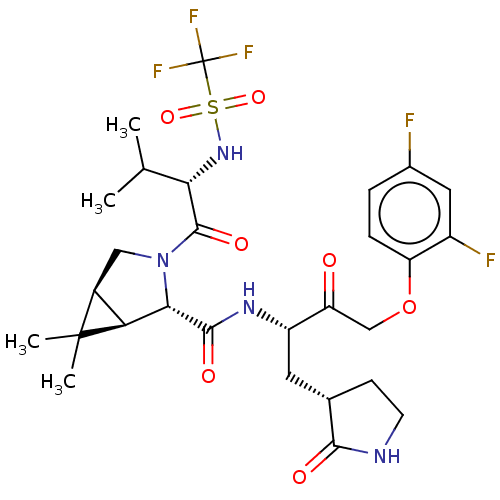 (WO2022208262, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 6.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 290: 551-60 (1999) BindingDB Entry DOI: 10.7270/Q2S18110 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581866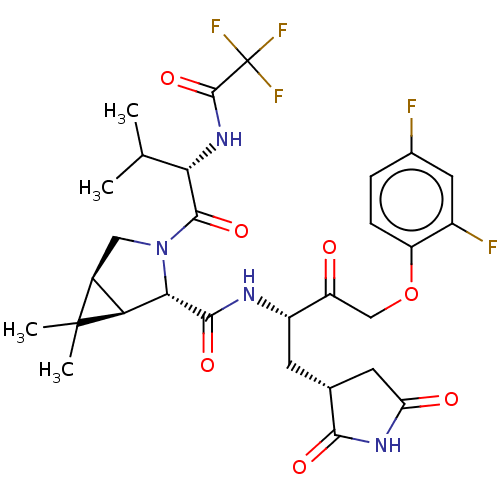 (WO2022208262, Example 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 7.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535126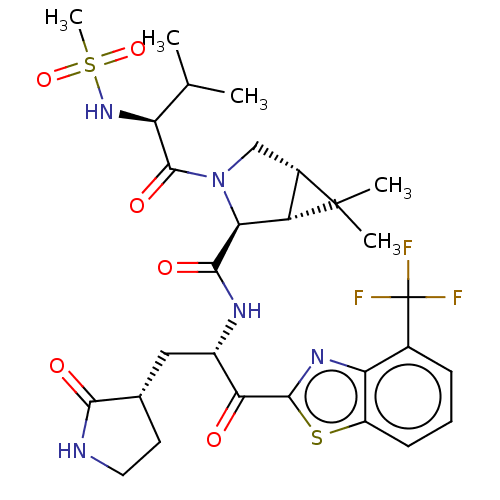 ((1R,2S,5S)-6,6-Dimethyl-3-[N-(methylsulfonyl)-L-va...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 7.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581867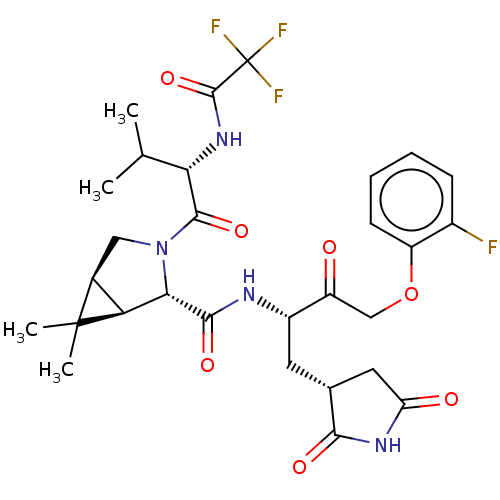 (WO2022208262, Example 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 7.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581844 (WO2022208262, Example 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 8.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581854 (WO2022208262, Example 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 8.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535168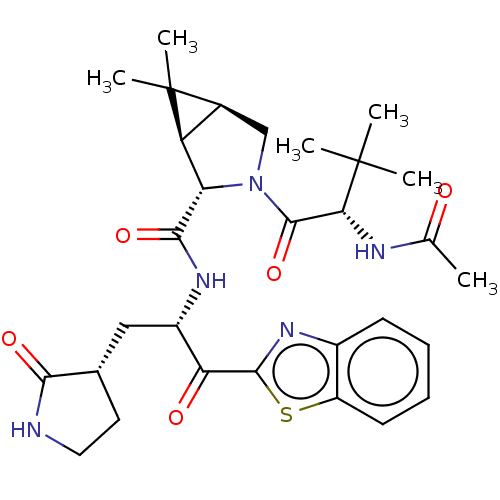 (WO2022013684, Example 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535164 (WO2022013684, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313979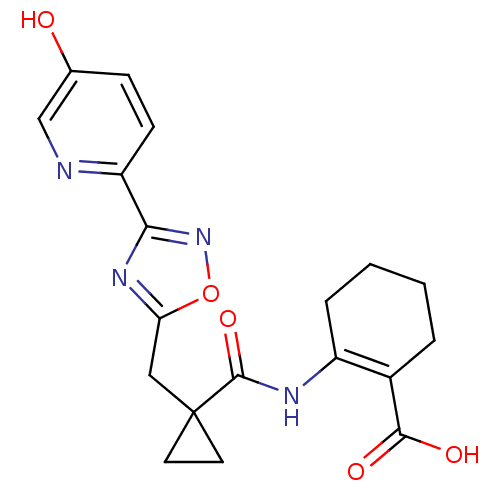 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581841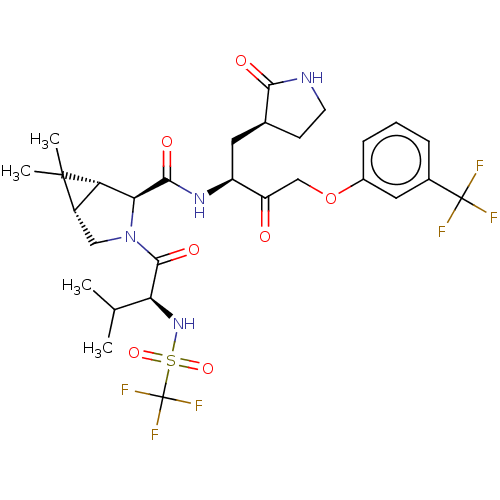 (WO2022208262, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535123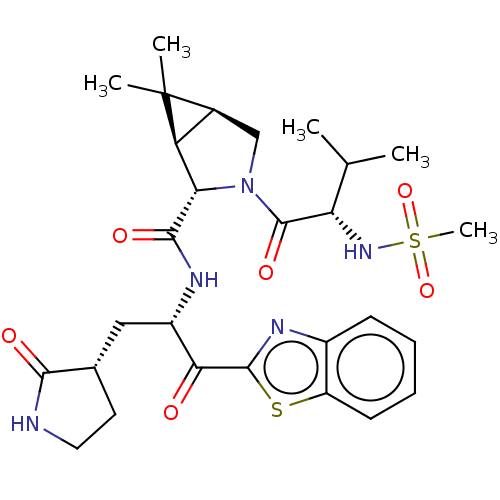 ((1R,2S,5S)-N-{(2S)-1-(1,3-Benzothiazol-2-yl)-1-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50132563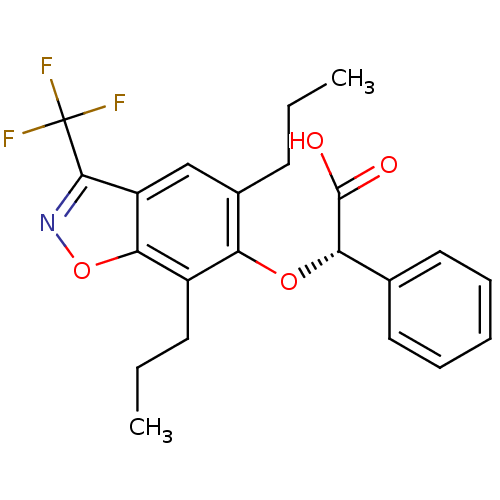 ((S)-(5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) | Bioorg Med Chem Lett 13: 3185-90 (2003) BindingDB Entry DOI: 10.7270/Q2TQ6229 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) was determined by HTRF assay | Bioorg Med Chem Lett 13: 3185-90 (2003) BindingDB Entry DOI: 10.7270/Q2TQ6229 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50132574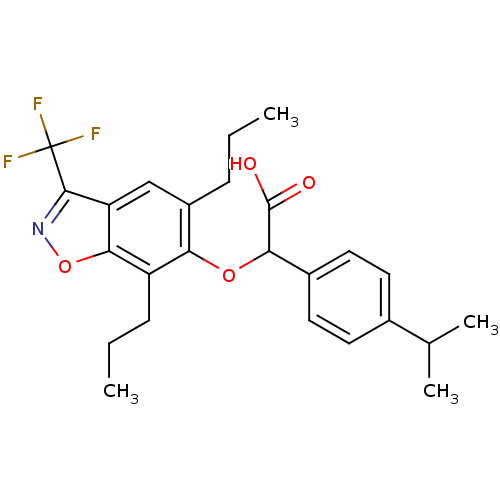 ((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay | Bioorg Med Chem Lett 13: 3185-90 (2003) BindingDB Entry DOI: 10.7270/Q2TQ6229 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535143 (WO2022013684, Example 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50132567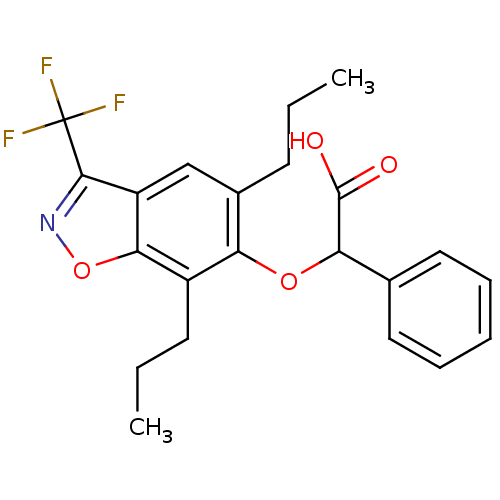 ((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay | Bioorg Med Chem Lett 13: 3185-90 (2003) BindingDB Entry DOI: 10.7270/Q2TQ6229 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535129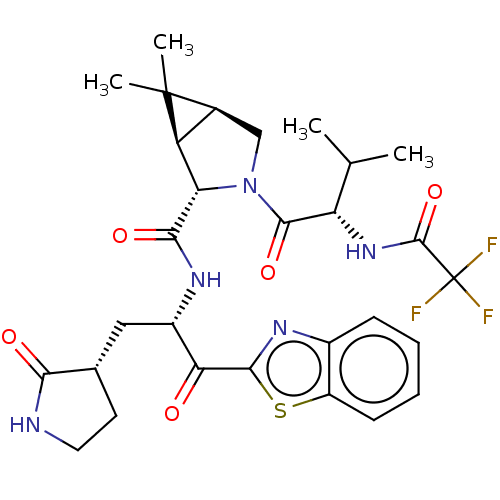 ((1R,2S,5S)-N-{(2S)-1-(1,3-Benzothiazol-2-yl)-1-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | WIPO WO2022013684 | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1829 total ) | Next | Last >> |