

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441002 (US10640486, Example 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441001 (US10640486, Example 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441010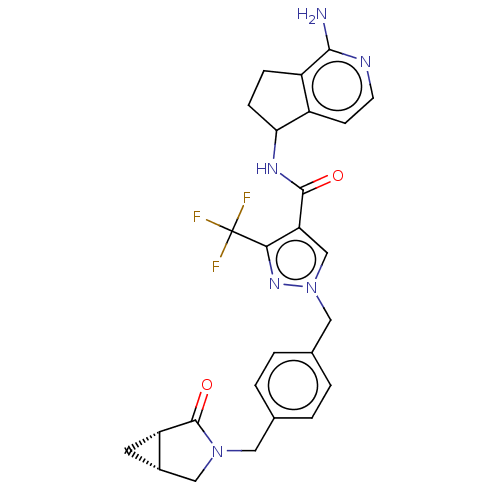 (US10640486, Example 50) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531034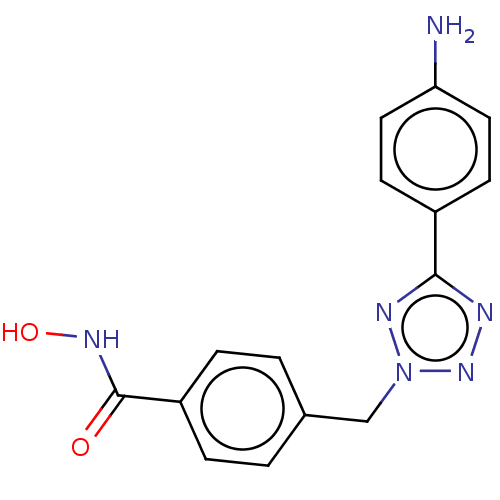 (CHEMBL4574641) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM440998 (US10640486, Example 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441037 (US10640486, Example 84) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531034 (CHEMBL4574641) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441047 (US10640486, Example 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM440996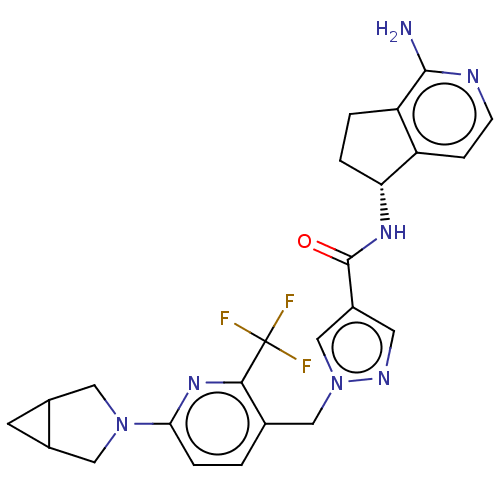 (US10640486, Example 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441028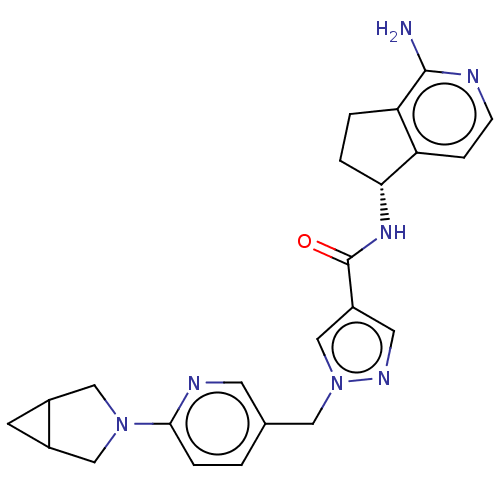 (1-[6-(3-Aza-bicyclo[3.1.0]hex-3-yl)-pyridin-3-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284757 (US10023575, Example 48 | US10023575, Example 49 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US10479794 (2019) BindingDB Entry DOI: 10.7270/Q27083TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM476570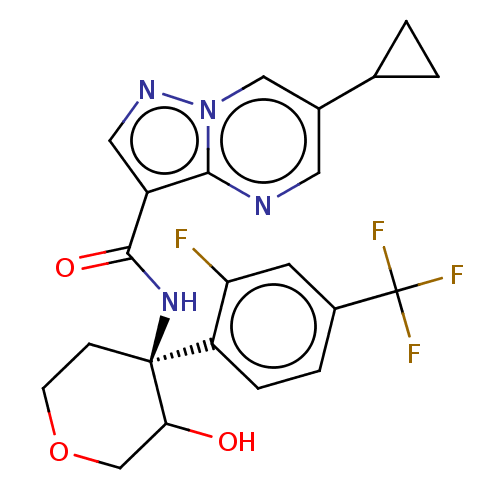 (US10875867, Example 82a | US11691977, Example 82b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US10875867 (2020) BindingDB Entry DOI: 10.7270/Q2WQ06W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM476574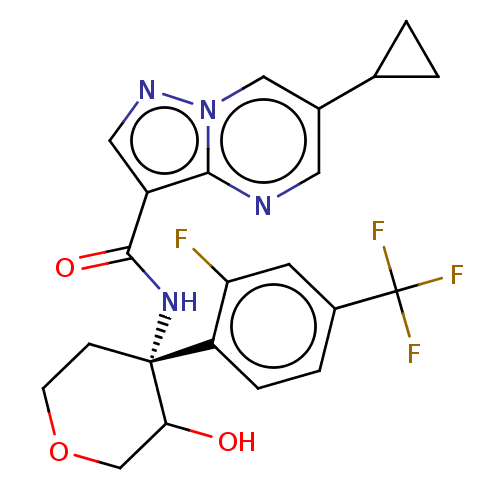 (US10875867, Example 82b | US11691977, Example 82a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26H4NHR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284757 (US10023575, Example 48 | US10023575, Example 49 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description All reactions were performed in 384 well plates, Perkin Elmer black optiplates and IMAP reaction buffer with 0.1% Tween20 (kit component)Compounds we... | US Patent US10023575 (2018) BindingDB Entry DOI: 10.7270/Q2KH0QCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284757 (US10023575, Example 48 | US10023575, Example 49 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26H4NHR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284757 (US10023575, Example 48 | US10023575, Example 49 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description All reactions were performed in 384 well plates, Perkin Elmer black optiplates and IMAP reaction buffer with 0.1% Tween20 (kit component)Compounds we... | US Patent US10023575 (2018) BindingDB Entry DOI: 10.7270/Q2KH0QCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284757 (US10023575, Example 48 | US10023575, Example 49 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US10479794 (2019) BindingDB Entry DOI: 10.7270/Q27083TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284757 (US10023575, Example 48 | US10023575, Example 49 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US10875867 (2020) BindingDB Entry DOI: 10.7270/Q2WQ06W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM139295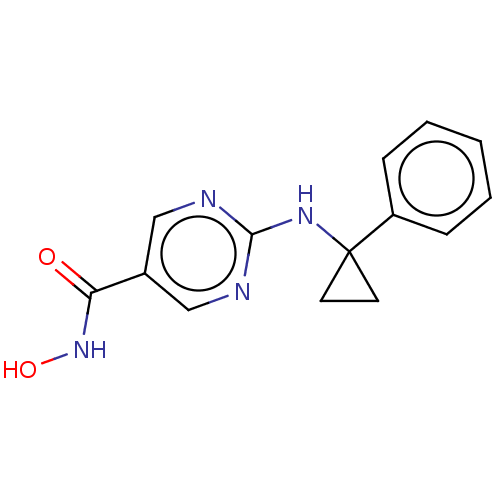 (US10858323, Compound 1 | US8614223, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531009 (CHEMBL4471726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM139295 (US10858323, Compound 1 | US8614223, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441030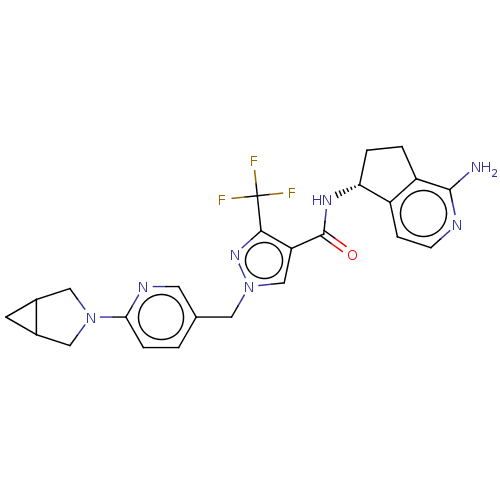 (US10640486, Example 77) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM440988 (US10640486, Example 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM440993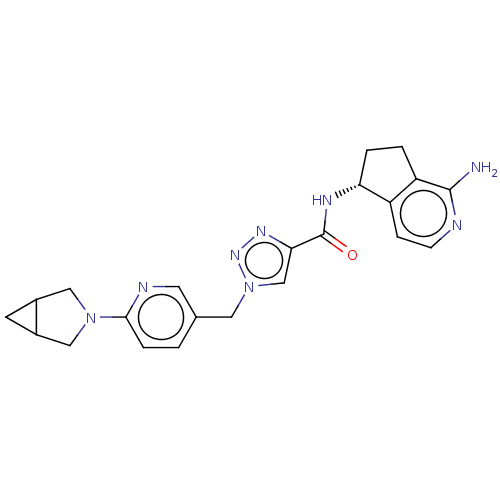 (US10640486, Example 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441008 (US10640486, Example 48 | US10640486, Example 49) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531009 (CHEMBL4471726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441041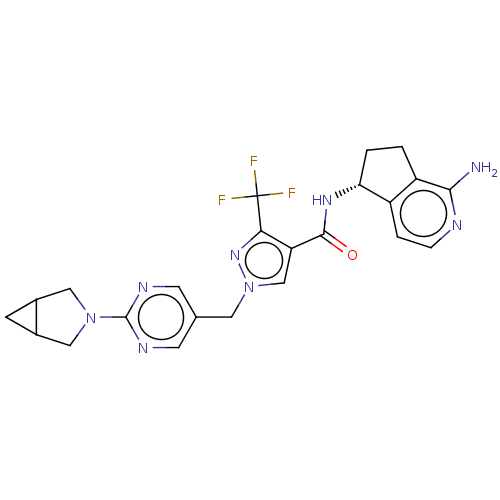 (1-[6-(3-Aza-bicyclo[3.1.0]hex-3-yl)-pyrimidin-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441033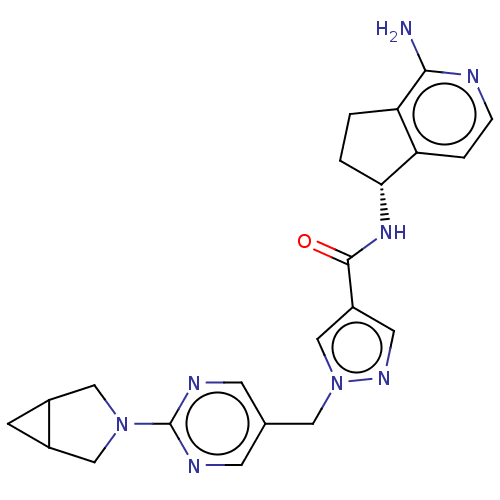 (US10640486, Example 80) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531007 (CHEMBL4568509) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531024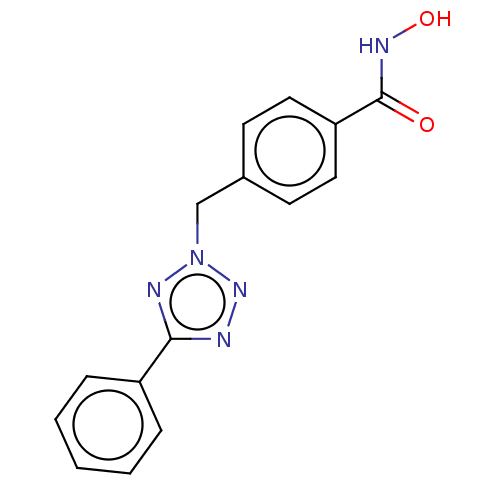 (CHEMBL4456695) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531008 (CHEMBL4458053) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531007 (CHEMBL4568509) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531024 (CHEMBL4456695) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531008 (CHEMBL4458053) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284755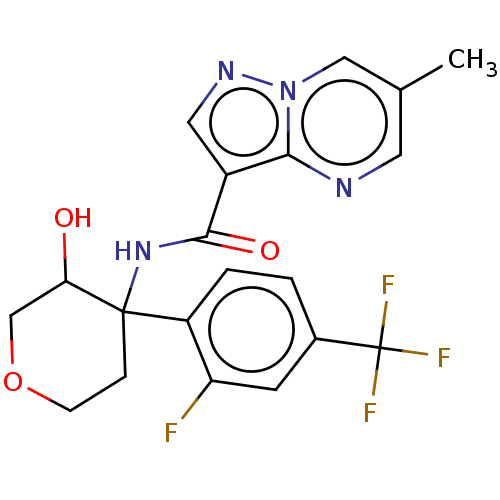 (US10023575, Example 46 | US10023575, Example 47 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description All reactions were performed in 384 well plates, Perkin Elmer black optiplates and IMAP reaction buffer with 0.1% Tween20 (kit component)Compounds we... | US Patent US10023575 (2018) BindingDB Entry DOI: 10.7270/Q2KH0QCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284755 (US10023575, Example 46 | US10023575, Example 47 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US10479794 (2019) BindingDB Entry DOI: 10.7270/Q27083TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM476669 (US10875867, Example 81a | US11691977, Example 81b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US10875867 (2020) BindingDB Entry DOI: 10.7270/Q2WQ06W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM476566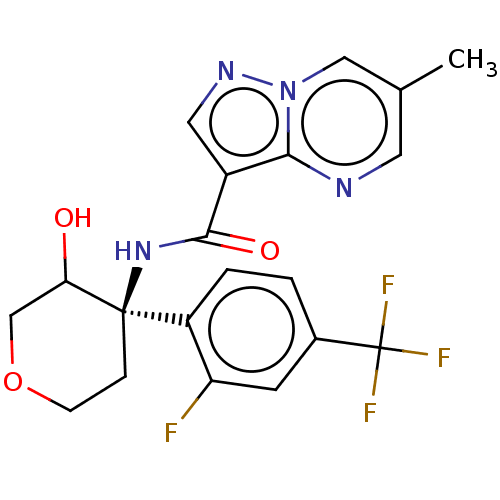 (US10875867, Example 81b | US11691977, Example 81a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26H4NHR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM476600 (US10875867, Example 86a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US10875867 (2020) BindingDB Entry DOI: 10.7270/Q2WQ06W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284762 (US10023575, Example 53 | US10023575, Example 54 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US10479794 (2019) BindingDB Entry DOI: 10.7270/Q27083TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284762 (US10023575, Example 53 | US10023575, Example 54 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description All reactions were performed in 384 well plates, Perkin Elmer black optiplates and IMAP reaction buffer with 0.1% Tween20 (kit component)Compounds we... | US Patent US10023575 (2018) BindingDB Entry DOI: 10.7270/Q2KH0QCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM608319 (US11691977, Example 85a | US11691977, Example 86a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26H4NHR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531044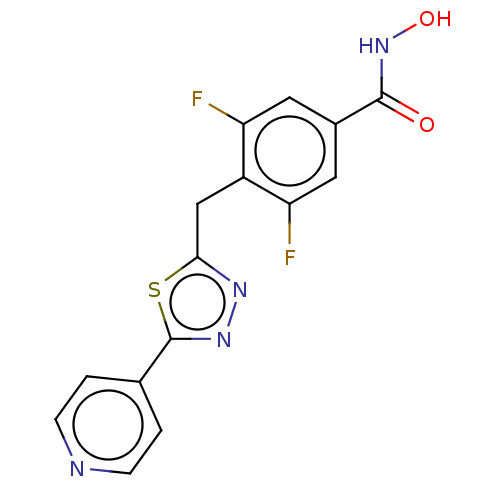 (CHEMBL4515782) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531023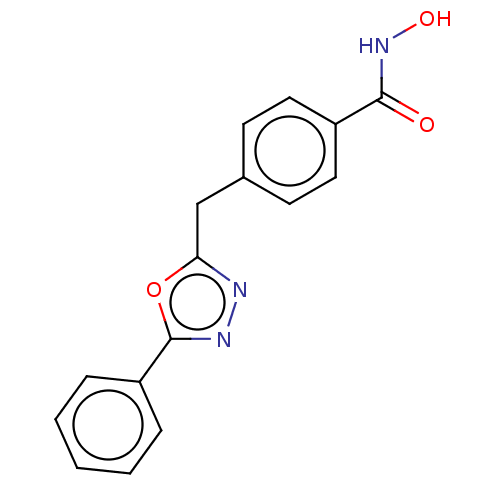 (CHEMBL4440425) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531039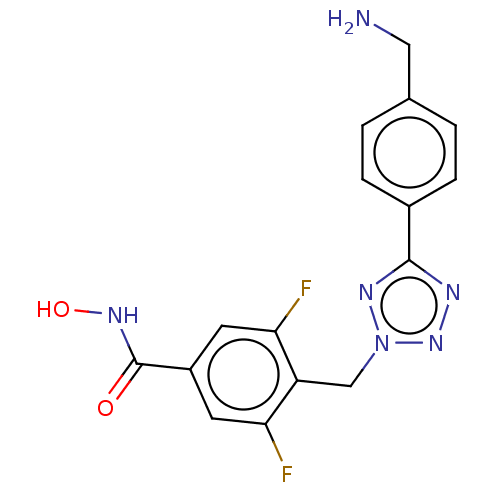 (CHEMBL4589682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441038 (US10640486, Example 85) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441007 (US10640486, Example 47) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531039 (CHEMBL4589682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531044 (CHEMBL4515782) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical R&D Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected sf9 insect cells pretreated with compound f... | J Med Chem 62: 10711-10739 (2019) Article DOI: 10.1021/acs.jmedchem.9b01194 BindingDB Entry DOI: 10.7270/Q2KP85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM440986 (US10640486, Example 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2209 total ) | Next | Last >> |