 Found 93 hits with Last Name = 'foster' and Initial = 'pa'
Found 93 hits with Last Name = 'foster' and Initial = 'pa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50539776

(CHEMBL4639677)Show InChI InChI=1S/C22H18N2O3/c1-2-3-6-11-26-17-12-16(14-24-10-9-23-15-24)21-20(13-17)27-19-8-5-4-7-18(19)22(21)25/h4-5,7-10,12-13,15H,2,11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50539777

(CHEMBL4632445)Show InChI InChI=1S/C22H18N2O3/c1-2-3-4-11-26-17-6-8-19-21(13-17)27-20-12-16(5-7-18(20)22(19)25)14-24-10-9-23-15-24/h5-10,12-13,15H,2,11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592781
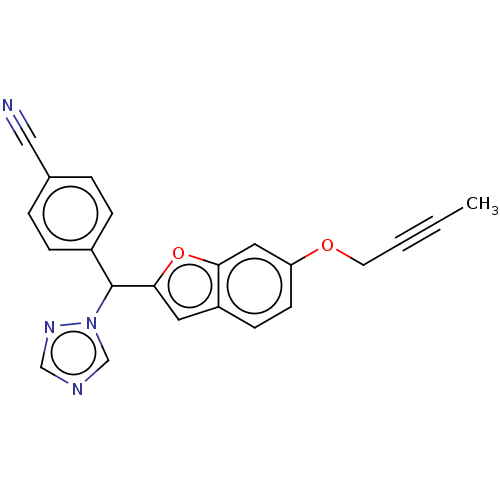
(CHEMBL5203413)Show SMILES CC#CCOc1ccc2cc(oc2c1)C(c1ccc(cc1)C#N)n1cncn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10007
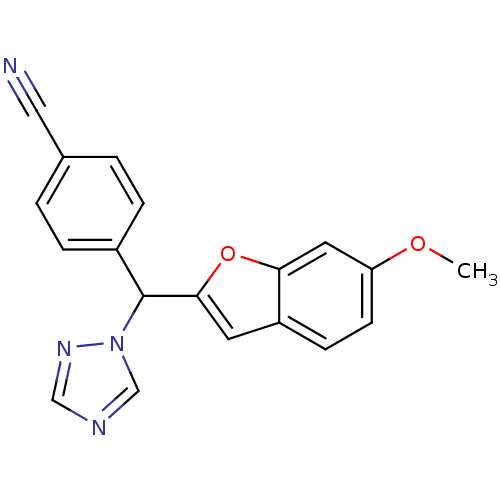
(4-[(6-methoxy-1-benzofuran-2-yl)(1H-1,2,4-triazol-...)Show InChI InChI=1S/C19H14N4O2/c1-24-16-7-6-15-8-18(25-17(15)9-16)19(23-12-21-11-22-23)14-4-2-13(10-20)3-5-14/h2-9,11-12,19H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10004

(1-[(4-fluorophenyl)(6-methoxy-1-benzofuran-2-yl)me...)Show InChI InChI=1S/C18H14FN3O2/c1-23-15-7-4-13-8-17(24-16(13)9-15)18(22-11-20-10-21-22)12-2-5-14(19)6-3-12/h2-11,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592778

(CHEMBL5175340) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50608157

(CHEMBL5271052)Show SMILES COc1ccc2cc(oc2c1)C(O)(c1ccc(Br)cc1)c1cccnc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592775

(CHEMBL5184004) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10001
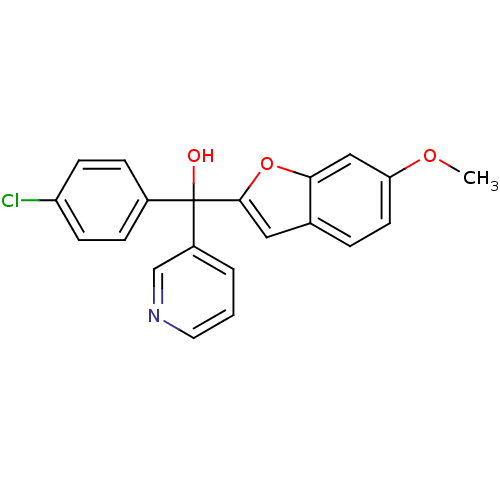
((4-chlorophenyl)(6-methoxy-1-benzofuran-2-yl)pyrid...)Show SMILES COc1ccc2cc(oc2c1)C(O)(c1ccc(Cl)cc1)c1cccnc1 Show InChI InChI=1S/C21H16ClNO3/c1-25-18-9-4-14-11-20(26-19(14)12-18)21(24,16-3-2-10-23-13-16)15-5-7-17(22)8-6-15/h2-13,24H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10005
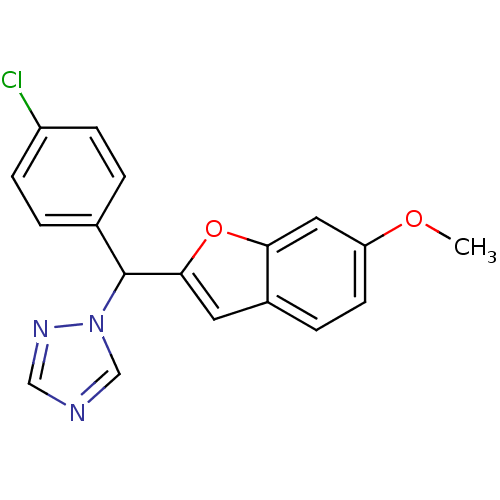
(1-[(4-chlorophenyl)(6-methoxy-1-benzofuran-2-yl)me...)Show InChI InChI=1S/C18H14ClN3O2/c1-23-15-7-4-13-8-17(24-16(13)9-15)18(22-11-20-10-21-22)12-2-5-14(19)6-3-12/h2-11,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592780
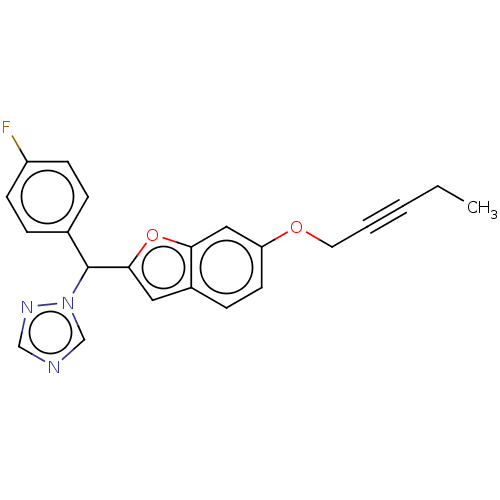
(CHEMBL5177269)Show SMILES CCC#CCOc1ccc2cc(oc2c1)C(c1ccc(F)cc1)n1cncn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592777
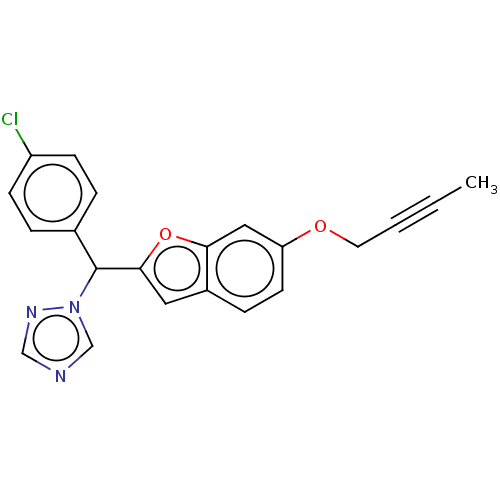
(CHEMBL5174567)Show SMILES CC#CCOc1ccc2cc(oc2c1)C(c1ccc(Cl)cc1)n1cncn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10013
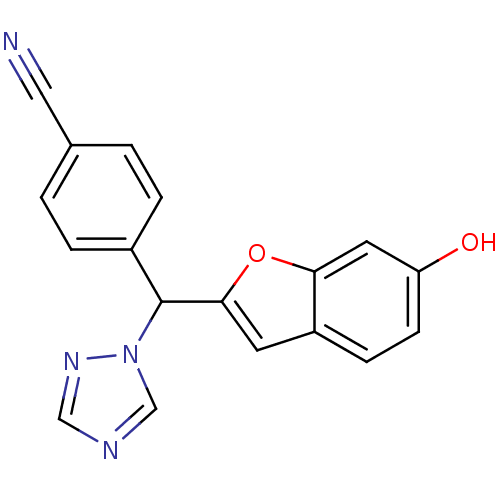
(4-[(6-Hydroxybenzofuran-2-yl)-[1,2,4]triazol-1-ylm...)Show InChI InChI=1S/C18H12N4O2/c19-9-12-1-3-13(4-2-12)18(22-11-20-10-21-22)17-7-14-5-6-15(23)8-16(14)24-17/h1-8,10-11,18,23H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM13061

(4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...)Show InChI InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM13061

(4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...)Show InChI InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592782

(CHEMBL5179009)Show SMILES CCC#CCOc1ccc2cc(oc2c1)C(c1ccc(cc1)C#N)n1cncn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10000
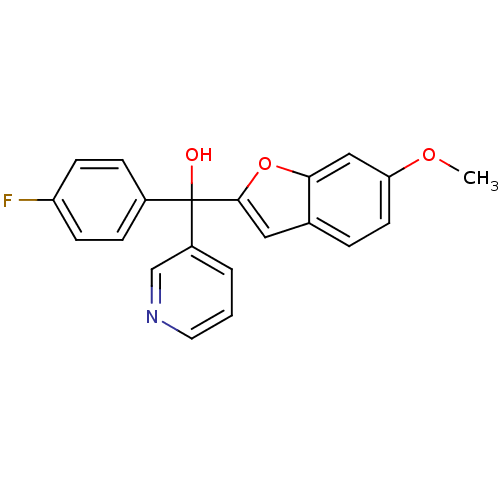
((4-fluorophenyl)(6-methoxy-1-benzofuran-2-yl)pyrid...)Show InChI InChI=1S/C21H16FNO3/c1-25-18-9-4-14-11-20(26-19(14)12-18)21(24,16-3-2-10-23-13-16)15-5-7-17(22)8-6-15/h2-13,24H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50539775
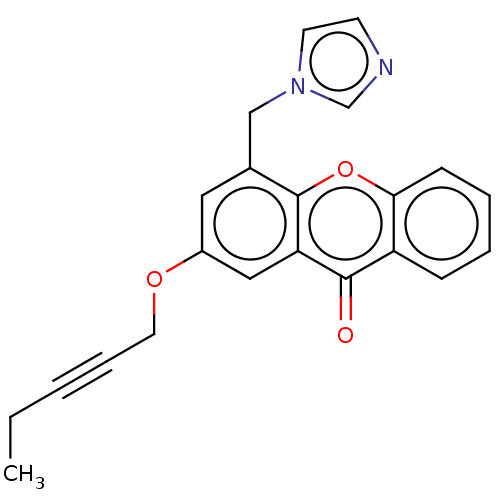
(CHEMBL4634777)Show InChI InChI=1S/C22H18N2O3/c1-2-3-6-11-26-17-12-16(14-24-10-9-23-15-24)22-19(13-17)21(25)18-7-4-5-8-20(18)27-22/h4-5,7-10,12-13,15H,2,11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10012
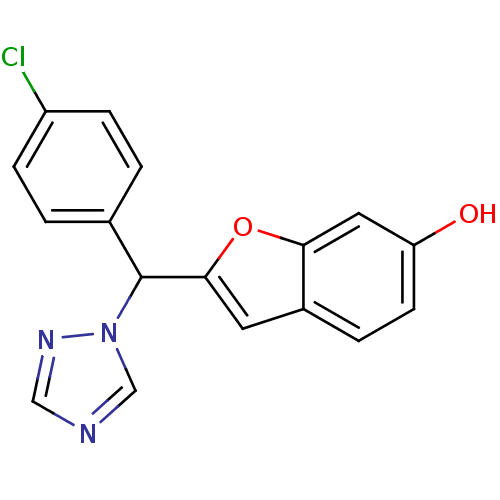
(2-[(4-Chlorophenyl)-[1,2,4]triazol-1-ylmethyl]benz...)Show InChI InChI=1S/C17H12ClN3O2/c18-13-4-1-11(2-5-13)17(21-10-19-9-20-21)16-7-12-3-6-14(22)8-15(12)23-16/h1-10,17,22H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50608161

(CHEMBL5283822)Show SMILES CC#CCOc1ccc2cc(oc2c1)C(O)(c1ccc(Br)cc1)c1cccnc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50608164
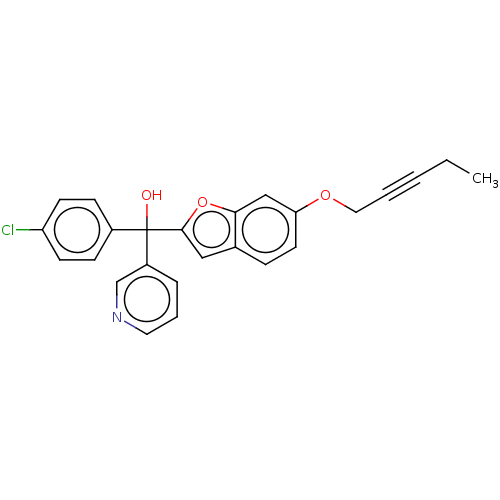
(CHEMBL5280326)Show SMILES CCC#CCOc1ccc2cc(oc2c1)C(O)(c1ccc(Cl)cc1)c1cccnc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592776
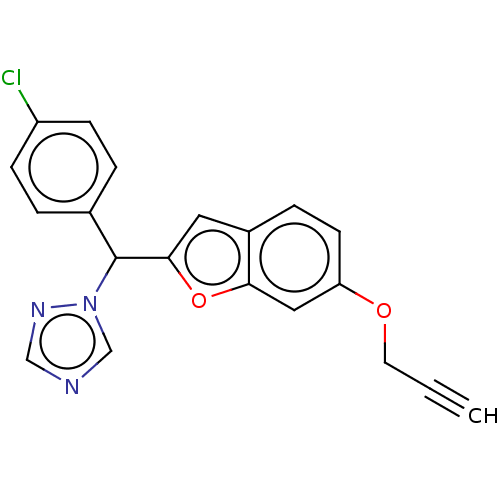
(CHEMBL5197552) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50608160

(CHEMBL5272700)Show SMILES CC#CCOc1ccc2cc(oc2c1)C(O)(c1ccc(Cl)cc1)c1cccnc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50167323
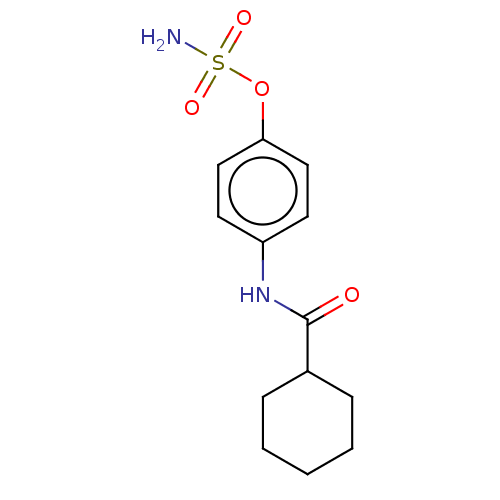
(CHEMBL3797224)Show InChI InChI=1S/C13H18N2O4S/c14-20(17,18)19-12-8-6-11(7-9-12)15-13(16)10-4-2-1-3-5-10/h6-10H,1-5H2,(H,15,16)(H2,14,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG-3 cells assessed as [14C]-Estrone formation using [3H]E1S as substrate |
Bioorg Med Chem 24: 2762-7 (2016)
Article DOI: 10.1016/j.bmc.2016.04.040
BindingDB Entry DOI: 10.7270/Q2XD13KC |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50608158
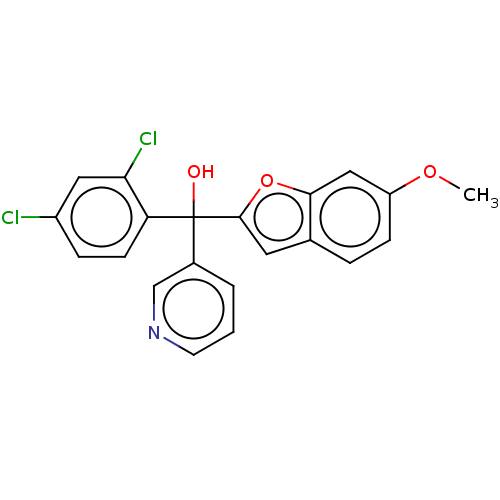
(CHEMBL5278036)Show SMILES COc1ccc2cc(oc2c1)C(O)(c1cccnc1)c1ccc(Cl)cc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592770
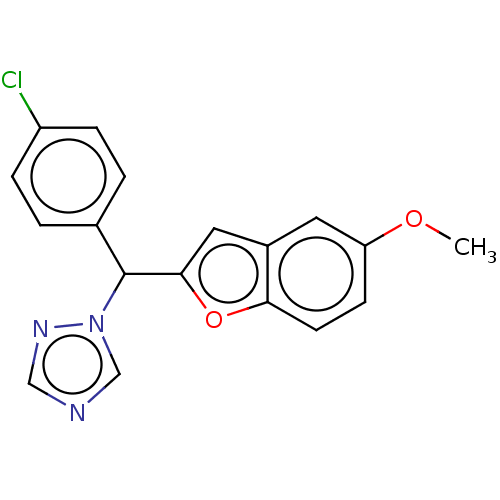
(CHEMBL5186519) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM13058
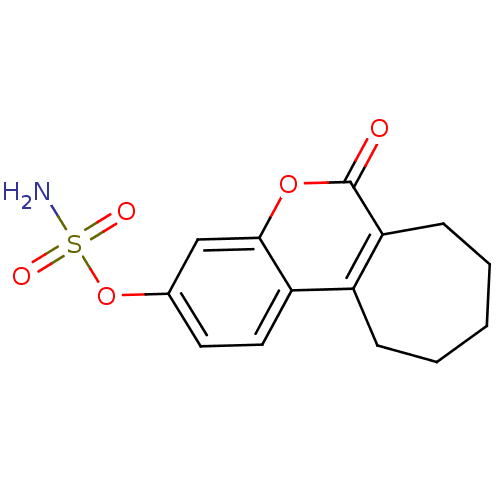
(6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...)Show InChI InChI=1S/C14H15NO5S/c15-21(17,18)20-9-6-7-11-10-4-2-1-3-5-12(10)14(16)19-13(11)8-9/h6-8H,1-5H2,(H2,15,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate incubated for 20 hrs by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592768
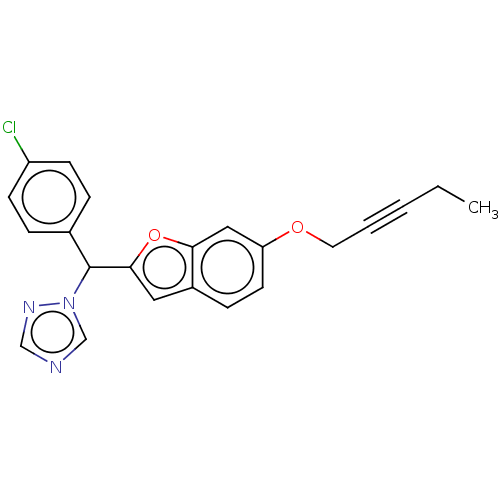
(CHEMBL5205534)Show SMILES CCC#CCOc1ccc2cc(oc2c1)C(c1ccc(Cl)cc1)n1cncn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592779
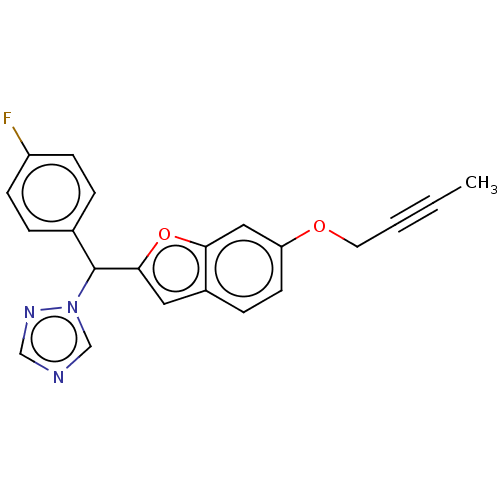
(CHEMBL5173597) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50608165
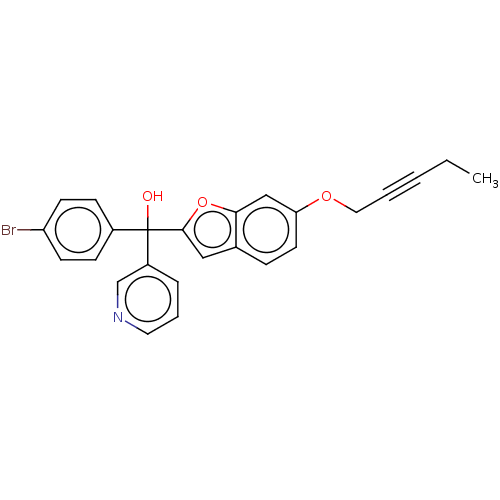
(CHEMBL5277776)Show SMILES CCC#CCOc1ccc2cc(oc2c1)C(O)(c1ccc(Br)cc1)c1cccnc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50528717
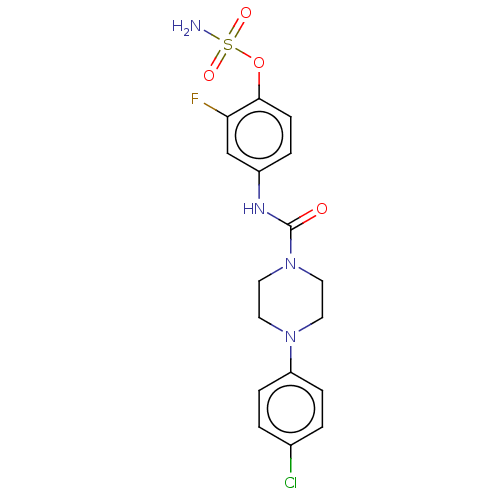
(CHEMBL4461896)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)N2CCN(CC2)c2ccc(Cl)cc2)cc1F Show InChI InChI=1S/C17H18ClFN4O4S/c18-12-1-4-14(5-2-12)22-7-9-23(10-8-22)17(24)21-13-3-6-16(15(19)11-13)27-28(20,25)26/h1-6,11H,7-10H2,(H,21,24)(H2,20,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111614
BindingDB Entry DOI: 10.7270/Q2VX0M04 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592769
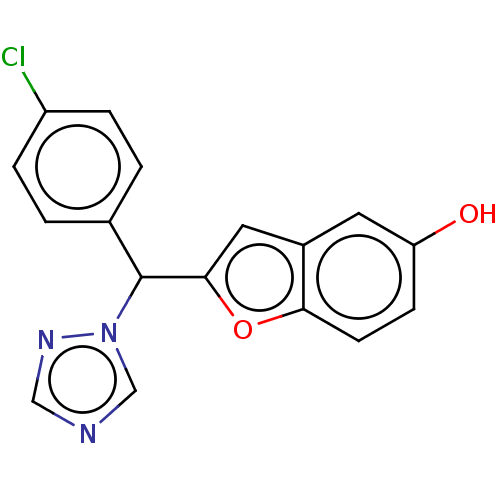
(CHEMBL5178202) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM13058

(6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...)Show InChI InChI=1S/C14H15NO5S/c15-21(17,18)20-9-6-7-11-10-4-2-1-3-5-12(10)14(16)19-13(11)8-9/h6-8H,1-5H2,(H2,15,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell lysates using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50528715

(CHEMBL4554860)Show SMILES CCCCCCCCN1CCN(CC1)C(=O)Nc1ccc(OS(N)(=O)=O)c(F)c1 Show InChI InChI=1S/C19H31FN4O4S/c1-2-3-4-5-6-7-10-23-11-13-24(14-12-23)19(25)22-16-8-9-18(17(20)15-16)28-29(21,26)27/h8-9,15H,2-7,10-14H2,1H3,(H,22,25)(H2,21,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111614
BindingDB Entry DOI: 10.7270/Q2VX0M04 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50608159
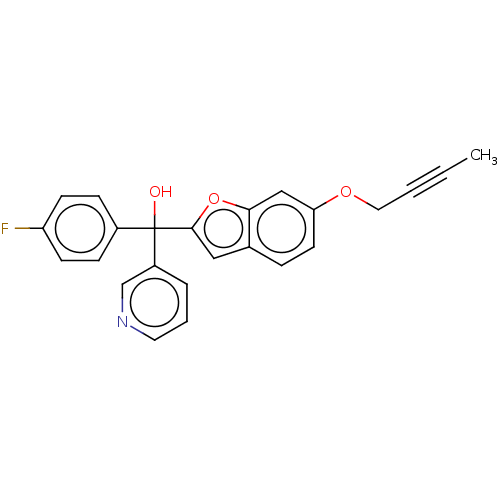
(CHEMBL5270023)Show SMILES CC#CCOc1ccc2cc(oc2c1)C(O)(c1ccc(F)cc1)c1cccnc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50608162
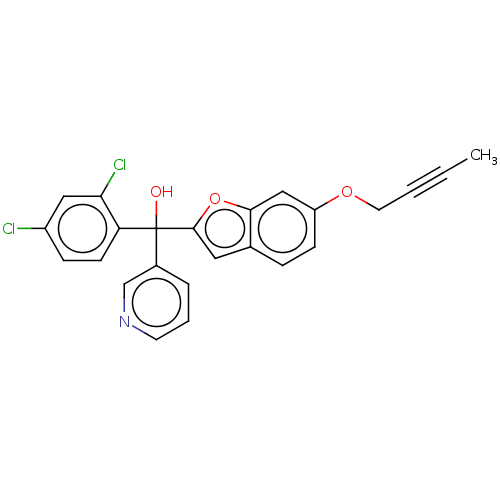
(CHEMBL5287457)Show SMILES CC#CCOc1ccc2cc(oc2c1)C(O)(c1cccnc1)c1ccc(Cl)cc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50608163
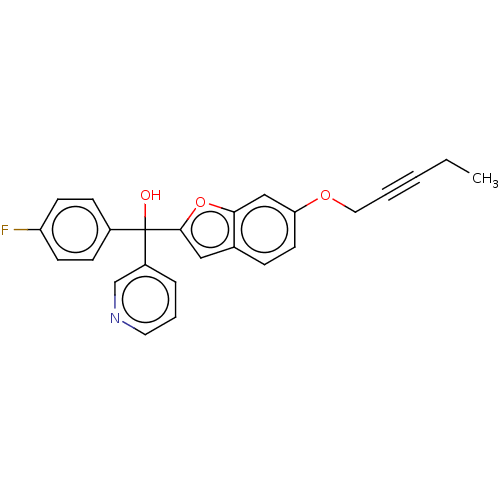
(CHEMBL5274335)Show SMILES CCC#CCOc1ccc2cc(oc2c1)C(O)(c1ccc(F)cc1)c1cccnc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50608166
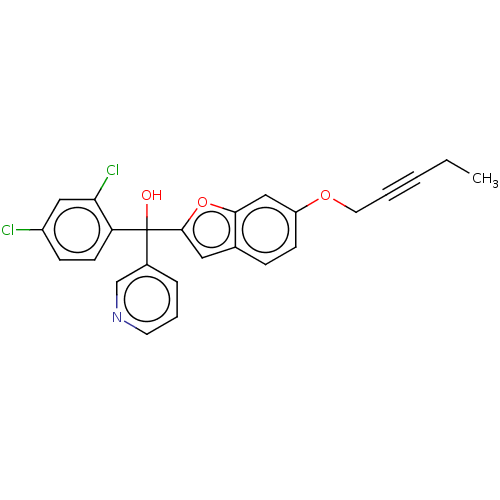
(CHEMBL5265932)Show SMILES CCC#CCOc1ccc2cc(oc2c1)C(O)(c1cccnc1)c1ccc(Cl)cc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50398452
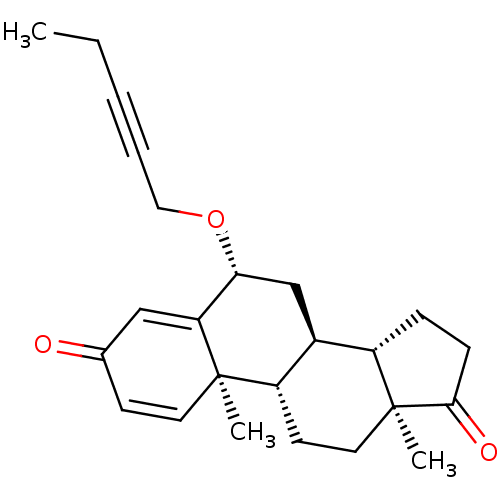
(CHEMBL2179110)Show SMILES CCC#CCO[C@@H]1C[C@H]2[C@@H]3CCC(=O)[C@@]3(C)CC[C@@H]2[C@@]2(C)C=CC(=O)C=C12 |r,c:23,t:27| Show InChI InChI=1S/C24H30O3/c1-4-5-6-13-27-21-15-17-18-7-8-22(26)24(18,3)12-10-19(17)23(2)11-9-16(25)14-20(21)23/h9,11,14,17-19,21H,4,7-8,10,12-13,15H2,1-3H3/t17-,18-,19-,21+,23+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50541451
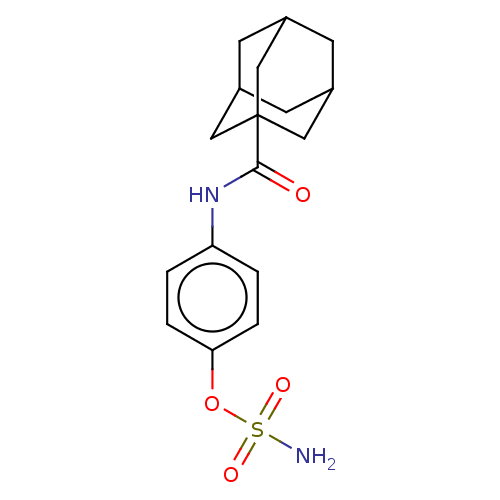
(CHEMBL4635151)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)C23CC4CC(CC(C4)C2)C3)cc1 |TLB:10:12:15:19.17.18,THB:17:16:13:19.18.20,17:18:15.16.21:13,20:18:15:21.12.13,20:12:15:19.17.18| Show InChI InChI=1S/C17H22N2O4S/c18-24(21,22)23-15-3-1-14(2-4-15)19-16(20)17-8-11-5-12(9-17)7-13(6-11)10-17/h1-4,11-13H,5-10H2,(H,19,20)(H2,18,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate incubated for 20 hrs by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50171448
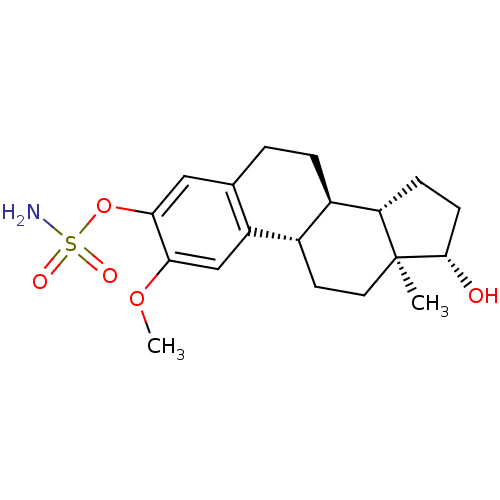
((9BETA,14BETA,17BETA)-17-HYDROXY-2-METHOXYESTRA-1,...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@@H](O)CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O Show InChI InChI=1S/C19H27NO5S/c1-19-8-7-12-13(15(19)5-6-18(19)21)4-3-11-9-17(25-26(20,22)23)16(24-2)10-14(11)12/h9-10,12-13,15,18,21H,3-8H2,1-2H3,(H2,20,22,23)/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase-mediated coversion of [3H]E1S to E1 |
J Med Chem 51: 1295-308 (2008)
Article DOI: 10.1021/jm701319c
BindingDB Entry DOI: 10.7270/Q2M0468S |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50528716
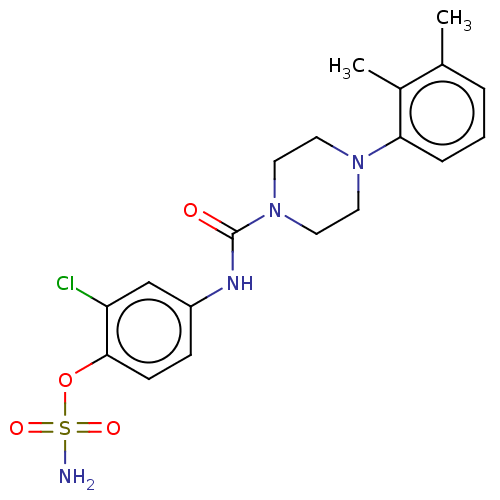
(CHEMBL4450604)Show SMILES Cc1cccc(N2CCN(CC2)C(=O)Nc2ccc(OS(N)(=O)=O)c(Cl)c2)c1C Show InChI InChI=1S/C19H23ClN4O4S/c1-13-4-3-5-17(14(13)2)23-8-10-24(11-9-23)19(25)22-15-6-7-18(16(20)12-15)28-29(21,26)27/h3-7,12H,8-11H2,1-2H3,(H,22,25)(H2,21,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111614
BindingDB Entry DOI: 10.7270/Q2VX0M04 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50528718
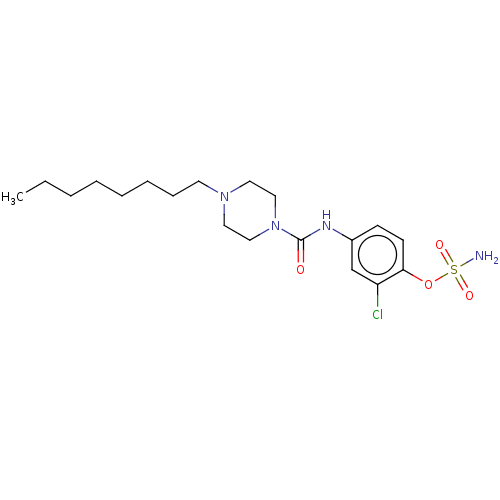
(CHEMBL4531739)Show SMILES CCCCCCCCN1CCN(CC1)C(=O)Nc1ccc(OS(N)(=O)=O)c(Cl)c1 Show InChI InChI=1S/C19H31ClN4O4S/c1-2-3-4-5-6-7-10-23-11-13-24(14-12-23)19(25)22-16-8-9-18(17(20)15-16)28-29(21,26)27/h8-9,15H,2-7,10-14H2,1H3,(H,22,25)(H2,21,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111614
BindingDB Entry DOI: 10.7270/Q2VX0M04 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM50592771
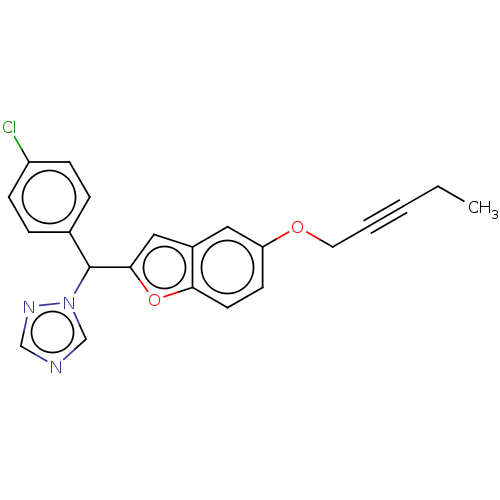
(CHEMBL5204674)Show SMILES CCC#CCOc1ccc2oc(cc2c1)C(c1ccc(Cl)cc1)n1cncn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50541453
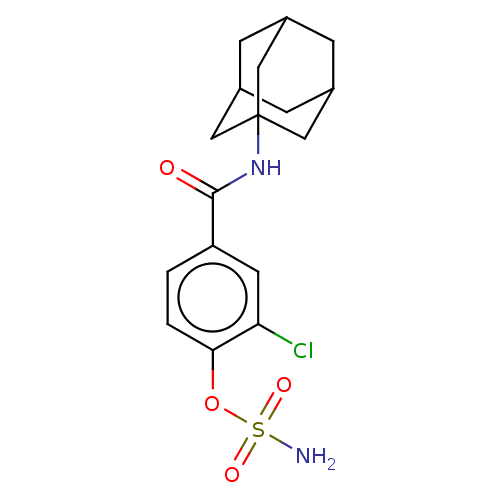
(CHEMBL4637433)Show SMILES NS(=O)(=O)Oc1ccc(cc1Cl)C(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:14:15:18:22.20.21,THB:20:19:16:22.21.23,20:21:18.19.24:16,23:21:18:24.15.16,23:15:18:22.20.21| Show InChI InChI=1S/C17H21ClN2O4S/c18-14-6-13(1-2-15(14)24-25(19,22)23)16(21)20-17-7-10-3-11(8-17)5-12(4-10)9-17/h1-2,6,10-12H,3-5,7-9H2,(H,20,21)(H2,19,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell lysates using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115406
BindingDB Entry DOI: 10.7270/Q2ZG6WSG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592772
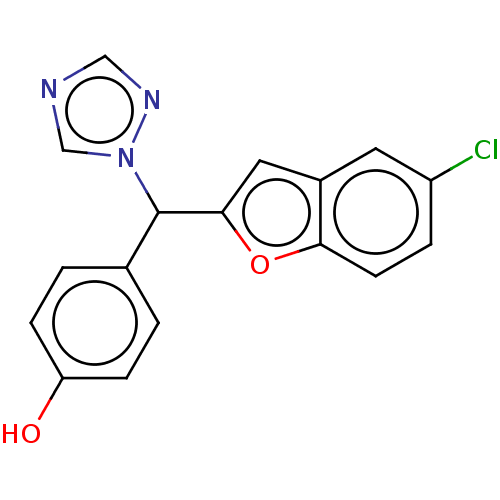
(CHEMBL5175025) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50528712
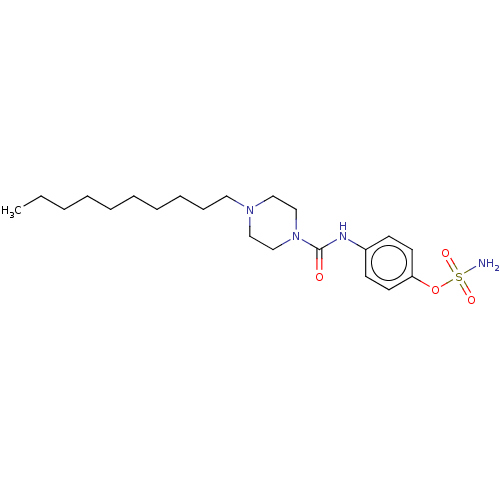
(CHEMBL4471248)Show SMILES CCCCCCCCCCN1CCN(CC1)C(=O)Nc1ccc(OS(N)(=O)=O)cc1 Show InChI InChI=1S/C21H36N4O4S/c1-2-3-4-5-6-7-8-9-14-24-15-17-25(18-16-24)21(26)23-19-10-12-20(13-11-19)29-30(22,27)28/h10-13H,2-9,14-18H2,1H3,(H,23,26)(H2,22,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG3 cell using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111614
BindingDB Entry DOI: 10.7270/Q2VX0M04 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50200936
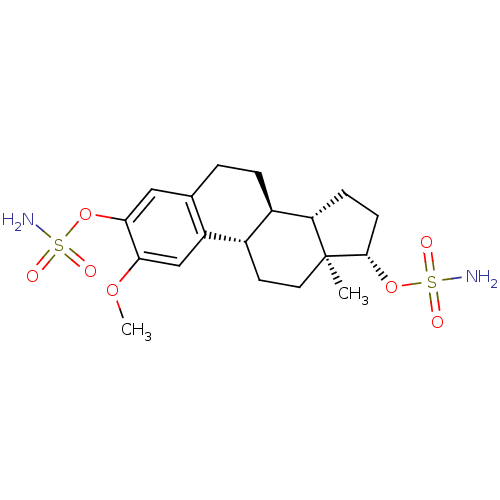
((9BETA,13ALPHA,14BETA,17ALPHA)-2-METHOXYESTRA-1,3,...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O)OS(N)(=O)=O |r| Show InChI InChI=1S/C19H28N2O7S2/c1-19-8-7-12-13(15(19)5-6-18(19)28-30(21,24)25)4-3-11-9-17(27-29(20,22)23)16(26-2)10-14(11)12/h9-10,12-13,15,18H,3-8H2,1-2H3,(H2,20,22,23)(H2,21,24,25)/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase-mediated coversion of [3H]E1S to E1 |
J Med Chem 51: 1295-308 (2008)
Article DOI: 10.1021/jm701319c
BindingDB Entry DOI: 10.7270/Q2M0468S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data