

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015743 (Acetic acid 3-[1-(3,6-dihydro-2H-pyridin-1-yl)-cyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015738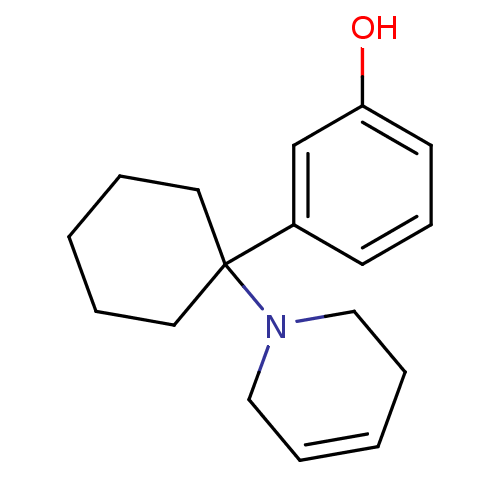 (3-[1-(3,6-Dihydro-2H-pyridin-1-yl)-cyclohexyl]-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015734 (1-(1-Thiophen-2-yl-cyclohexyl)-1,2,3,6-tetrahydro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015741 (1-[1-(3-Methoxy-phenyl)-cyclohexyl]-1,2,3,6-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015732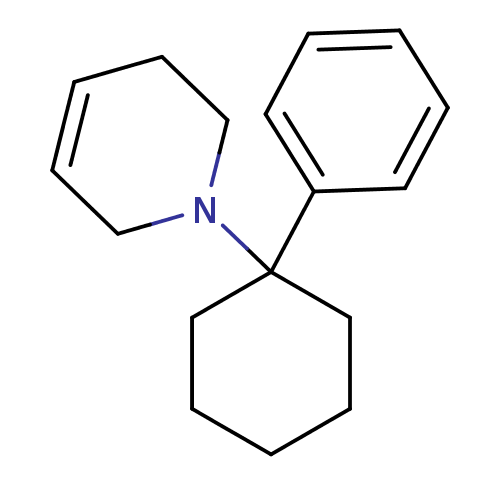 (1-(1-Phenyl-cyclohexyl)-1,2,3,6-tetrahydro-pyridin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015733 (3-[1-(3,6-Dihydro-2H-pyridin-1-yl)-cyclohexyl]-ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015735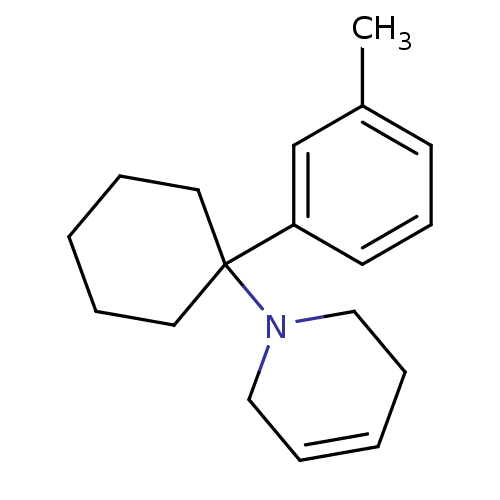 (1-(1-m-Tolyl-cyclohexyl)-1,2,3,6-tetrahydro-pyridi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015744 (1-[1-(3-Methoxymethoxy-phenyl)-cyclohexyl]-1,2,3,6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015740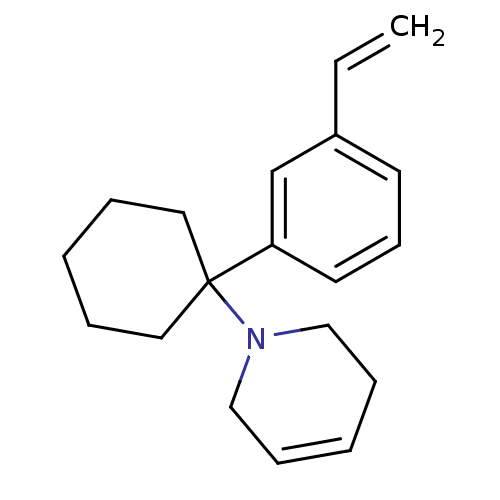 (1-[1-(3-Vinyl-phenyl)-cyclohexyl]-1,2,3,6-tetrahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015731 (1-[1-(3-Methylsulfanyl-phenyl)-cyclohexyl]-1,2,3,6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015736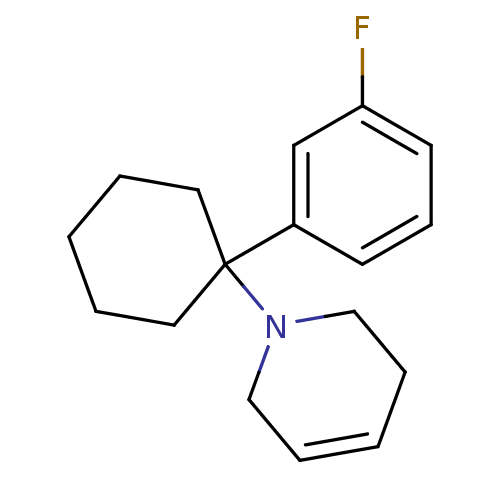 (1-[1-(3-Fluoro-phenyl)-cyclohexyl]-1,2,3,6-tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015739 (3-[1-(3,6-Dihydro-2H-pyridin-1-yl)-cyclohexyl]-ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015730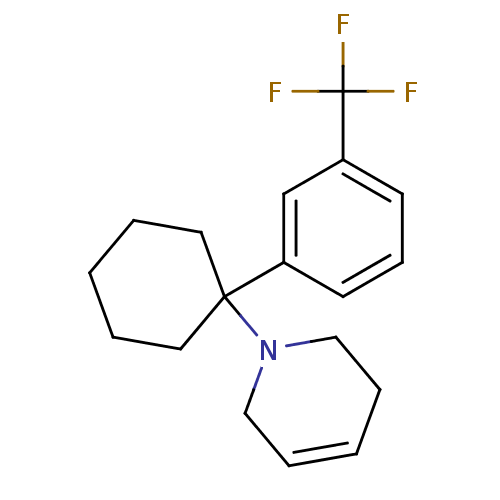 (1-[1-(3-Trifluoromethyl-phenyl)-cyclohexyl]-1,2,3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 422 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015742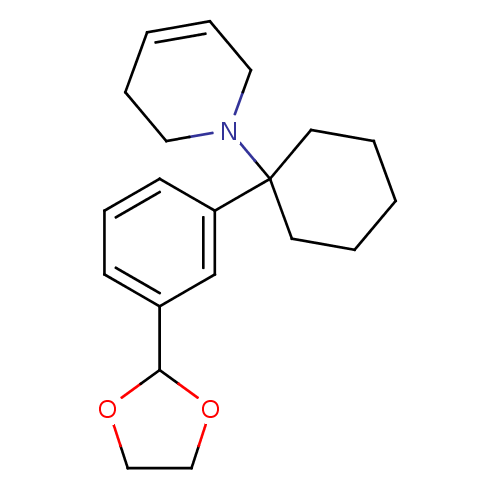 (1-[1-(3-[1,3]Dioxolan-2-yl-phenyl)-cyclohexyl]-1,2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 601 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015745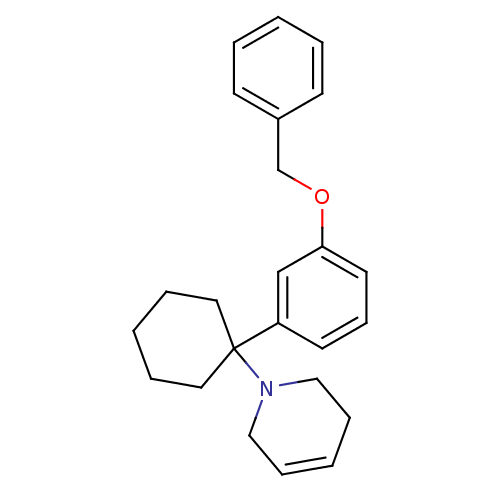 (1-[1-(3-Benzyloxy-phenyl)-cyclohexyl]-1,2,3,6-tetr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 733 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM86149 (CAS_131413 | NCS-382 | NSC_131413) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 675-9 (2003) Article DOI: 10.1124/jpet.102.046797 BindingDB Entry DOI: 10.7270/Q2S1812F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015737 (1-[1-(3,5-Dimethoxy-phenyl)-cyclohexyl]-1,2,3,6-te...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM86151 (UMB68) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 675-9 (2003) Article DOI: 10.1124/jpet.102.046797 BindingDB Entry DOI: 10.7270/Q2S1812F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM86152 (UMB75) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 675-9 (2003) Article DOI: 10.1124/jpet.102.046797 BindingDB Entry DOI: 10.7270/Q2S1812F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM86150 (UMB58) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 675-9 (2003) Article DOI: 10.1124/jpet.102.046797 BindingDB Entry DOI: 10.7270/Q2S1812F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM50168281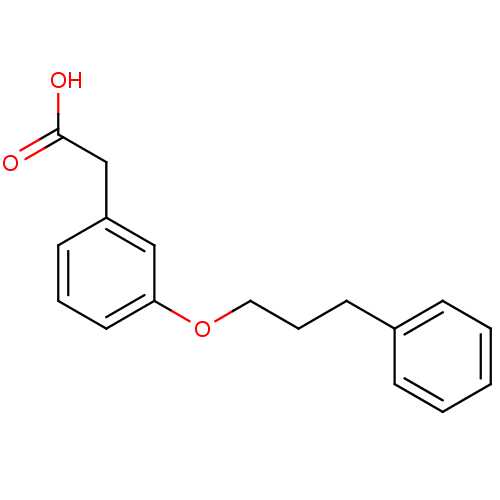 (CHEMBL189853 | [3-(3-Phenyl-propoxy)-phenyl]-aceti...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]-NCS-382 (16 nM) from GHB receptor of rat cerebrocortical membranes | Bioorg Med Chem Lett 15: 3201-2 (2005) Article DOI: 10.1016/j.bmcl.2005.05.011 BindingDB Entry DOI: 10.7270/Q2MP52TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM50168285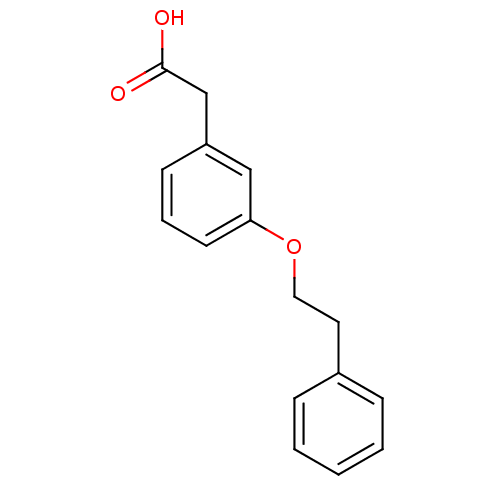 ((3-Phenethyloxy-phenyl)-acetic acid | CHEMBL190961) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]-NCS-382 (16 nM) from GHB receptor of rat cerebrocortical membranes | Bioorg Med Chem Lett 15: 3201-2 (2005) Article DOI: 10.1016/j.bmcl.2005.05.011 BindingDB Entry DOI: 10.7270/Q2MP52TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM50168283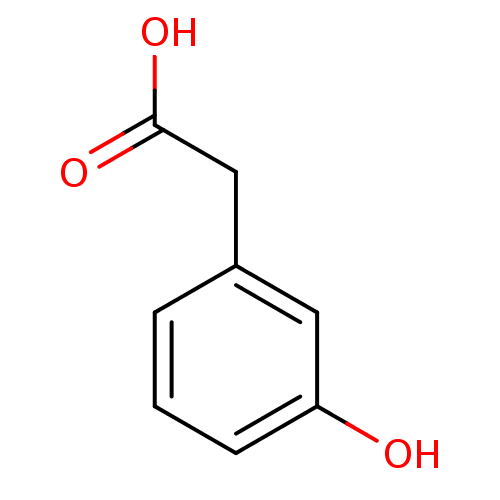 ((3-hydroxyphenyl)acetic acid | (m-hydroxyphenyl)ac...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]-NCS-382 (16 nM) from GHB receptor of rat cerebrocortical membranes | Bioorg Med Chem Lett 15: 3201-2 (2005) Article DOI: 10.1016/j.bmcl.2005.05.011 BindingDB Entry DOI: 10.7270/Q2MP52TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM50060976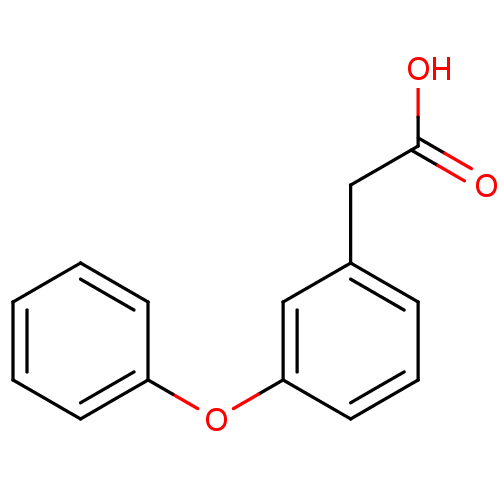 ((3-Phenoxy-phenyl)-acetic acid | CHEMBL107066) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]-NCS-382 (16 nM) from GHB receptor of rat cerebrocortical membranes | Bioorg Med Chem Lett 15: 3201-2 (2005) Article DOI: 10.1016/j.bmcl.2005.05.011 BindingDB Entry DOI: 10.7270/Q2MP52TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM50023575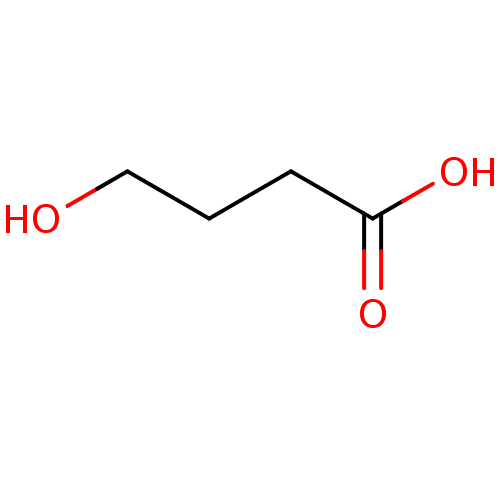 (4-hydroxy-butyric acid | 4-hydroxybutanoic acid | ...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]-NCS-382 (16 nM) from GHB receptor of rat cerebrocortical membranes | Bioorg Med Chem Lett 15: 3201-2 (2005) Article DOI: 10.1016/j.bmcl.2005.05.011 BindingDB Entry DOI: 10.7270/Q2MP52TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM50168282 ((3-Benzyloxy-phenyl)-acetic acid | CHEMBL190962) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]-NCS-382 (16 nM) from GHB receptor of rat cerebrocortical membranes | Bioorg Med Chem Lett 15: 3201-2 (2005) Article DOI: 10.1016/j.bmcl.2005.05.011 BindingDB Entry DOI: 10.7270/Q2MP52TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-hydroxybutyrate receptor (RAT) | BDBM50168284 (4-Phenoxy-butyric acid | CHEMBL190723) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]-NCS-382 (16 nM) from GHB receptor of rat cerebrocortical membranes | Bioorg Med Chem Lett 15: 3201-2 (2005) Article DOI: 10.1016/j.bmcl.2005.05.011 BindingDB Entry DOI: 10.7270/Q2MP52TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||