 Found 42 hits with Last Name = 'frankshun' and Initial = 'r'
Found 42 hits with Last Name = 'frankshun' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50016904

(CHEMBL3273012)Show SMILES C[C@H](NC(C)=O)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)NN(C)C(=O)O[C@@H](C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C25H36N6O7/c1-15(27-18(4)32)21(33)28-16(2)24(36)31-13-9-12-20(31)23(35)29-30(5)25(37)38-17(3)22(34)26-14-19-10-7-6-8-11-19/h6-8,10-11,15-17,20H,9,12-14H2,1-5H3,(H,26,34)(H,27,32)(H,28,33)(H,29,35)/t15-,16-,17-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis |
J Med Chem 20: 1464-8 (1977)
BindingDB Entry DOI: 10.7270/Q2WQ05BG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50016905
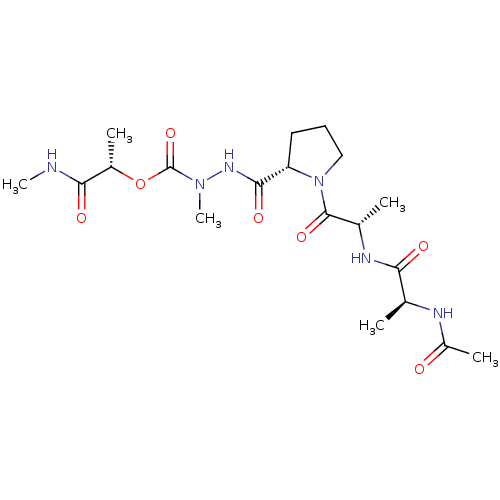
(CHEMBL3273010)Show SMILES CNC(=O)[C@H](C)OC(=O)N(C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](C)NC(C)=O |r| Show InChI InChI=1S/C19H32N6O7/c1-10(21-13(4)26)15(27)22-11(2)18(30)25-9-7-8-14(25)17(29)23-24(6)19(31)32-12(3)16(28)20-5/h10-12,14H,7-9H2,1-6H3,(H,20,28)(H,21,26)(H,22,27)(H,23,29)/t10-,11-,12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis |
J Med Chem 20: 1464-8 (1977)
BindingDB Entry DOI: 10.7270/Q2WQ05BG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50016903
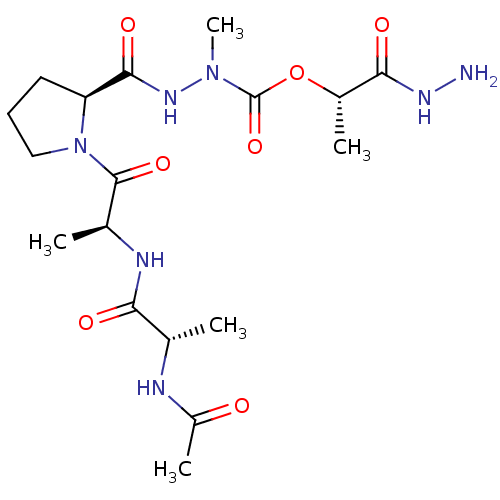
(CHEMBL3273013)Show SMILES C[C@H](NC(C)=O)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)NN(C)C(=O)O[C@@H](C)C(=O)NN |r| Show InChI InChI=1S/C18H31N7O7/c1-9(20-12(4)26)14(27)21-10(2)17(30)25-8-6-7-13(25)16(29)23-24(5)18(31)32-11(3)15(28)22-19/h9-11,13H,6-8,19H2,1-5H3,(H,20,26)(H,21,27)(H,22,28)(H,23,29)/t9-,10-,11-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis |
J Med Chem 20: 1464-8 (1977)
BindingDB Entry DOI: 10.7270/Q2WQ05BG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50016929
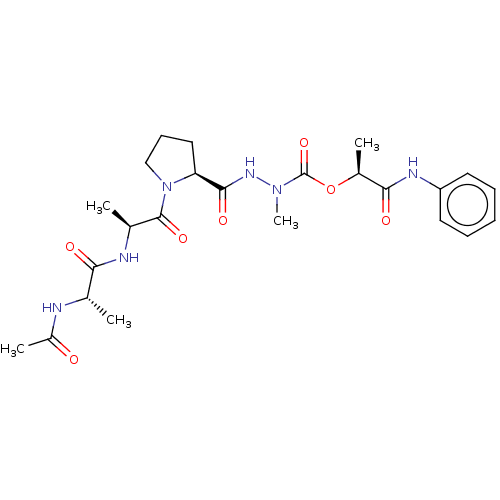
(CHEMBL3273011)Show SMILES C[C@H](NC(C)=O)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)NN(C)C(=O)O[C@@H](C)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C24H34N6O7/c1-14(25-17(4)31)20(32)26-15(2)23(35)30-13-9-12-19(30)22(34)28-29(5)24(36)37-16(3)21(33)27-18-10-7-6-8-11-18/h6-8,10-11,14-16,19H,9,12-13H2,1-5H3,(H,25,31)(H,26,32)(H,27,33)(H,28,34)/t14-,15-,16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis |
J Med Chem 20: 1464-8 (1977)
BindingDB Entry DOI: 10.7270/Q2WQ05BG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50016930
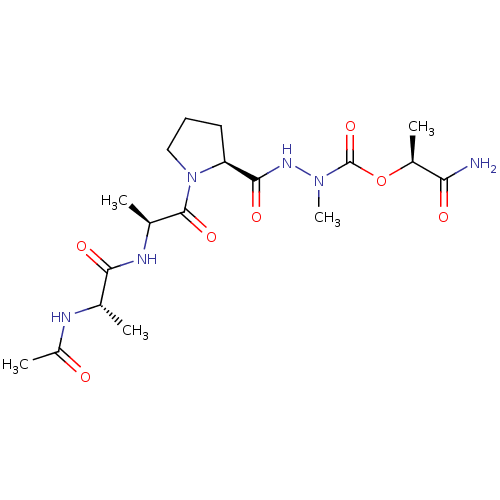
(CHEMBL3273008)Show SMILES C[C@H](NC(C)=O)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)NN(C)C(=O)O[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C18H30N6O7/c1-9(20-12(4)25)15(27)21-10(2)17(29)24-8-6-7-13(24)16(28)22-23(5)18(30)31-11(3)14(19)26/h9-11,13H,6-8H2,1-5H3,(H2,19,26)(H,20,25)(H,21,27)(H,22,28)/t9-,10-,11-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis |
J Med Chem 20: 1464-8 (1977)
BindingDB Entry DOI: 10.7270/Q2WQ05BG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50016906
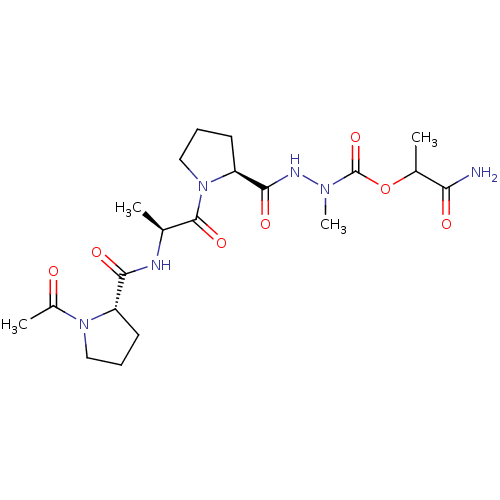
(CHEMBL3273009)Show SMILES C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N1CCC[C@H]1C(=O)NN(C)C(=O)OC(C)C(N)=O |r| Show InChI InChI=1S/C20H32N6O7/c1-11(22-17(29)14-7-5-9-25(14)13(3)27)19(31)26-10-6-8-15(26)18(30)23-24(4)20(32)33-12(2)16(21)28/h11-12,14-15H,5-10H2,1-4H3,(H2,21,28)(H,22,29)(H,23,30)/t11-,12?,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis |
J Med Chem 20: 1464-8 (1977)
BindingDB Entry DOI: 10.7270/Q2WQ05BG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase Jak 2 |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Tyrosine kinase 2 kinase |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase Jak 3 |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Mus musculus) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of murine Jak 1 protein kinase |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226348
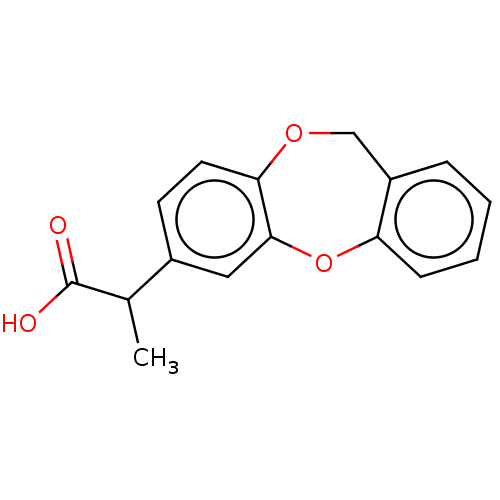
(CHEMBL284705)Show InChI InChI=1S/C16H14O4/c1-10(16(17)18)11-6-7-14-15(8-11)20-13-5-3-2-4-12(13)9-19-14/h2-8,10H,9H2,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226339
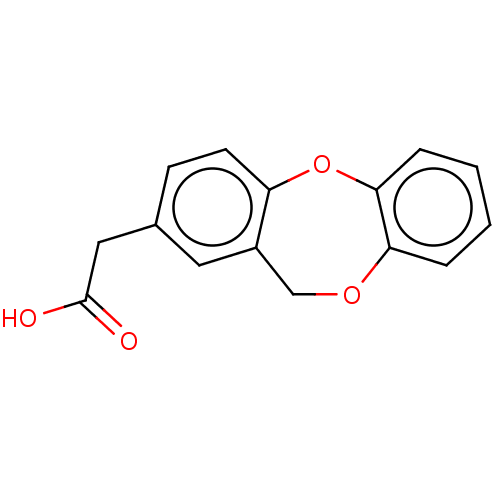
(CHEMBL33378)Show InChI InChI=1S/C15H12O4/c16-15(17)8-10-5-6-12-11(7-10)9-18-13-3-1-2-4-14(13)19-12/h1-7H,8-9H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mitogen-activated protein kinase ERK |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of I-kappa-B kinase 2 |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226341
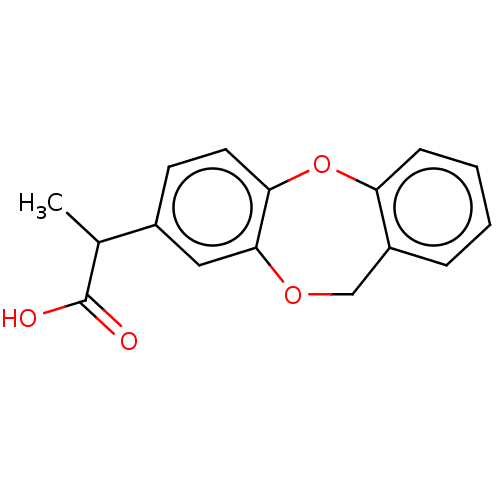
(CHEMBL33987)Show InChI InChI=1S/C16H14O4/c1-10(16(17)18)11-6-7-14-15(8-11)19-9-12-4-2-3-5-13(12)20-14/h2-8,10H,9H2,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase Fyn |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 3 |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitin of Fibroblast growth factor receptor 2 |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C alpha |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226346
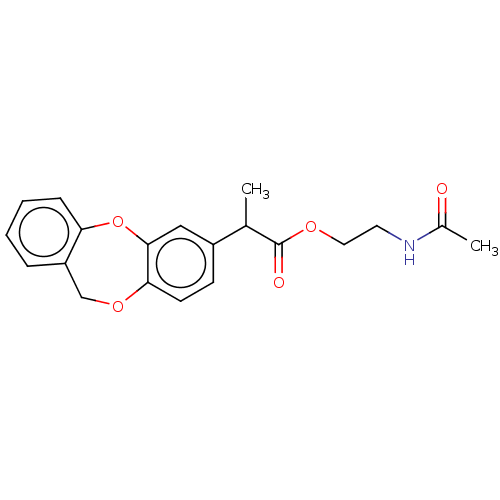
(CHEMBL34003)Show InChI InChI=1S/C20H21NO5/c1-13(20(23)24-10-9-21-14(2)22)15-7-8-18-19(11-15)26-17-6-4-3-5-16(17)12-25-18/h3-8,11,13H,9-10,12H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2 |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226349
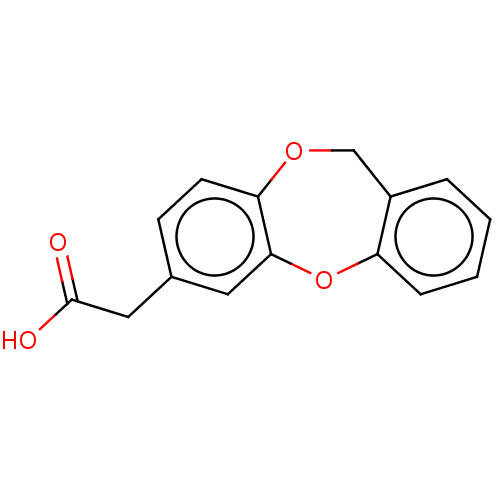
(CHEMBL32961)Show InChI InChI=1S/C15H12O4/c16-15(17)8-10-5-6-13-14(7-10)19-12-4-2-1-3-11(12)9-18-13/h1-7H,8-9H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226342
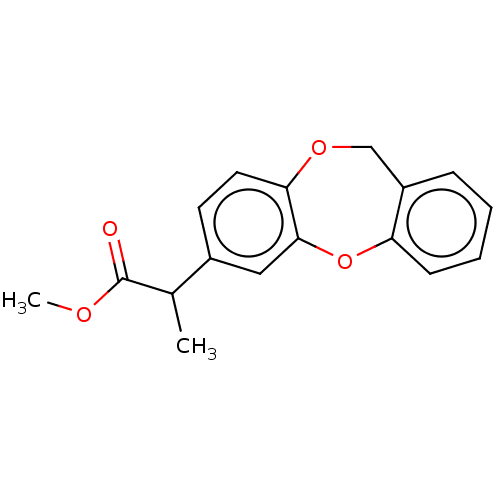
(CHEMBL285188)Show InChI InChI=1S/C17H16O4/c1-11(17(18)19-2)12-7-8-15-16(9-12)21-14-6-4-3-5-13(14)10-20-15/h3-9,11H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1/2/3/4
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Fibroblast growth factor receptor |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 1 |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226343
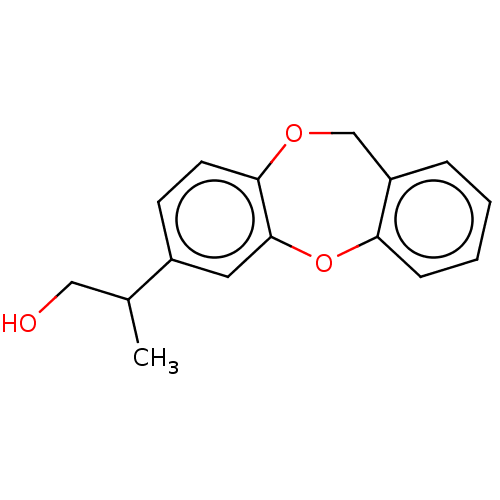
(CHEMBL32990)Show InChI InChI=1S/C16H16O3/c1-11(9-17)12-6-7-15-16(8-12)19-14-5-3-2-4-13(14)10-18-15/h2-8,11,17H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Tyrosine-protein kinase CSK |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cyclin dependent kinase 2 |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226340
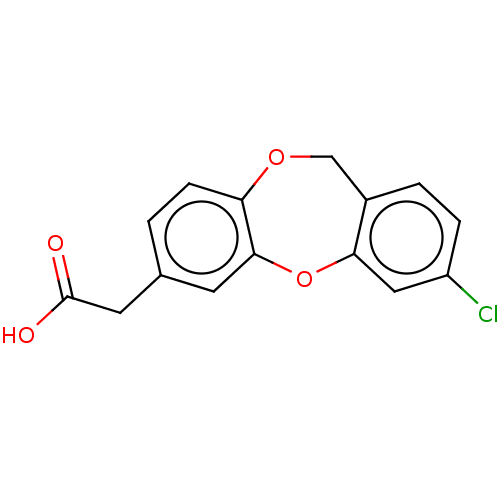
(CHEMBL286802)Show InChI InChI=1S/C15H11ClO4/c16-11-3-2-10-8-19-12-4-1-9(6-15(17)18)5-14(12)20-13(10)7-11/h1-5,7H,6,8H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase Hck |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226345
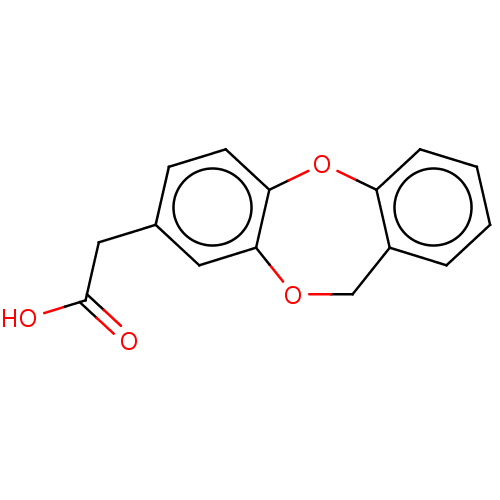
(CHEMBL33814)Show InChI InChI=1S/C15H12O4/c16-15(17)8-10-5-6-13-14(7-10)18-9-11-3-1-2-4-12(11)19-13/h1-7H,8-9H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase Raf |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Tek kinase |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226344
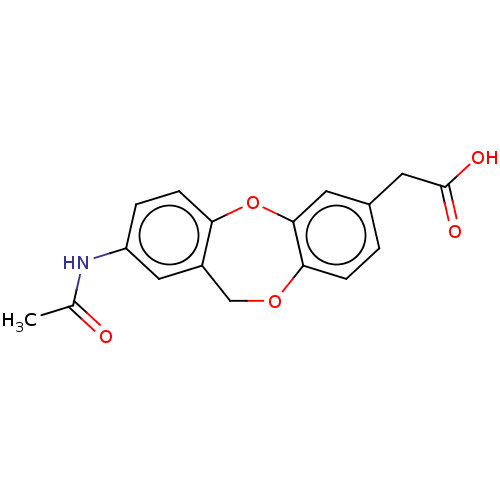
(CHEMBL32218)Show InChI InChI=1S/C17H15NO5/c1-10(19)18-13-3-5-14-12(8-13)9-22-15-4-2-11(7-17(20)21)6-16(15)23-14/h2-6,8H,7,9H2,1H3,(H,18,19)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Ovis aries (Sheep)) | BDBM50226347
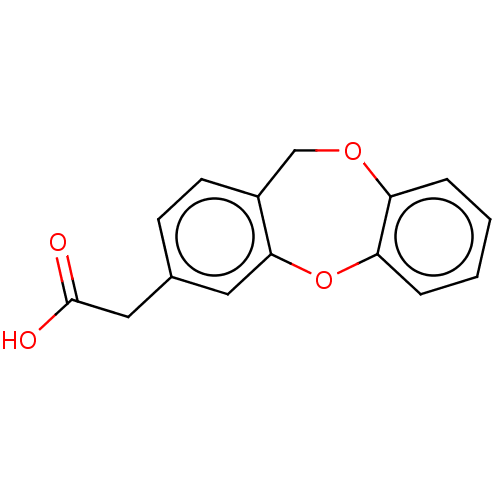
(CHEMBL33527)Show InChI InChI=1S/C15H12O4/c16-15(17)8-10-5-6-11-9-18-12-3-1-2-4-13(12)19-14(11)7-10/h1-7H,8-9H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes |
J Med Chem 29: 1436-41 (1986)
BindingDB Entry DOI: 10.7270/Q2X06974 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data