

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146155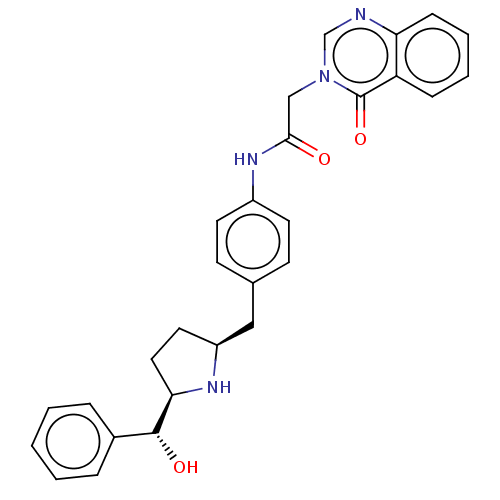 (CHEMBL3764774) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50092645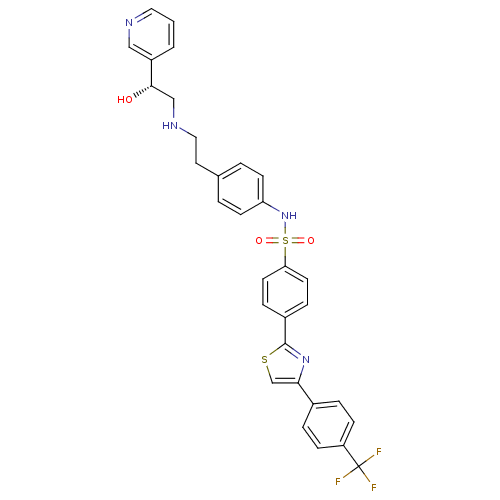 ((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG channel | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50448787 (CHEMBL3128178) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50092645 ((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146157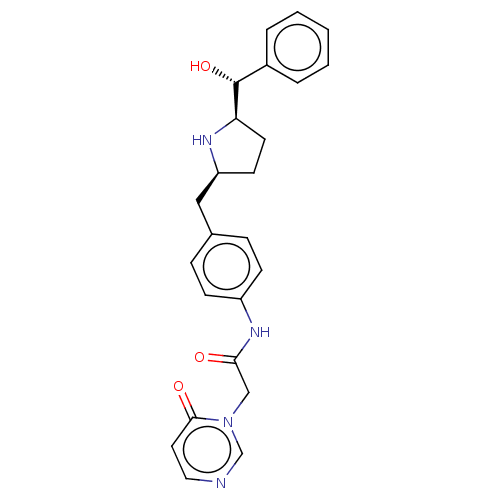 (CHEMBL3764950) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146161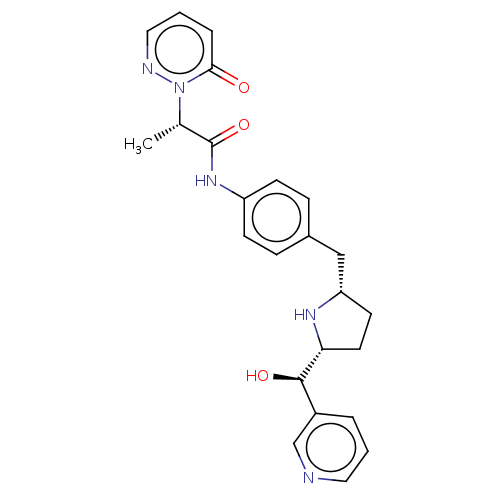 (CHEMBL3763998) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50448789 (CHEMBL3128188) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50092645 ((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146159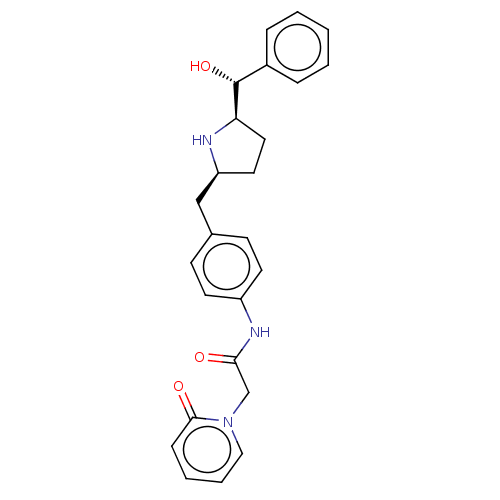 (CHEMBL3764592) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50338821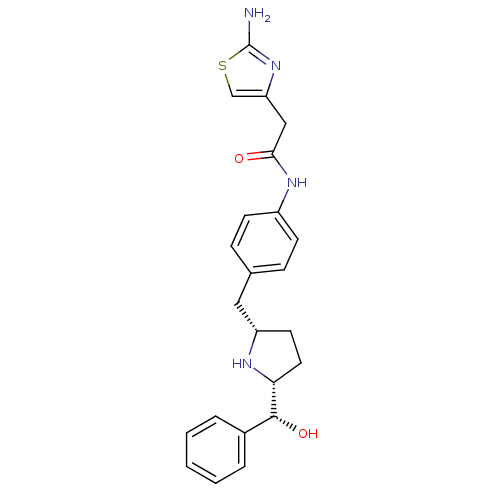 (2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50092645 ((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-diltiazem from human Cav1.2 channel | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50146155 (CHEMBL3764774) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG channel | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50146155 (CHEMBL3764774) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50338821 (2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes assessed as dextraomethorphan O-demethylation | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146158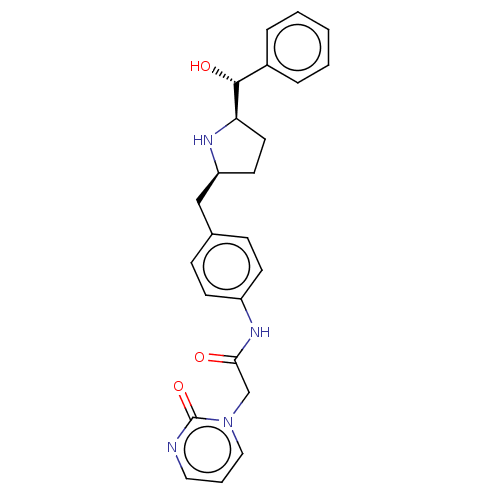 (CHEMBL3763594) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146156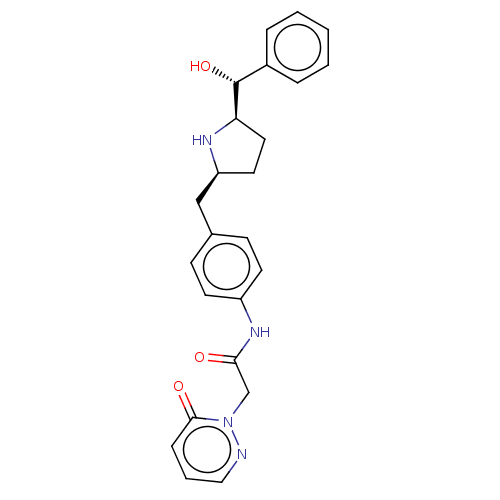 (CHEMBL3764088) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50146162 (CHEMBL3763934) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human dopamine transporter | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50092645 ((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50448787 (CHEMBL3128178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG channel | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146162 (CHEMBL3763934) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50338821 (2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-diltiazem from human Cav1.2 channel | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50092645 ((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146160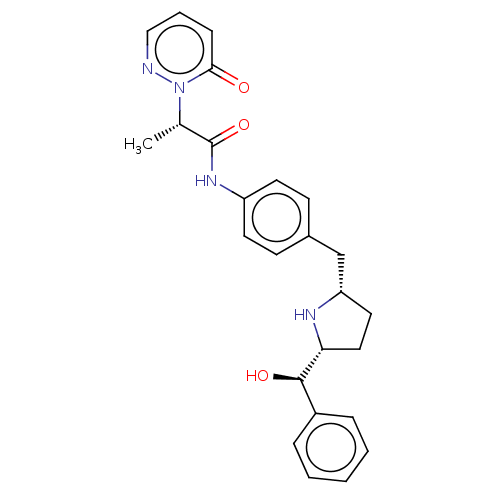 (CHEMBL3765335) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146154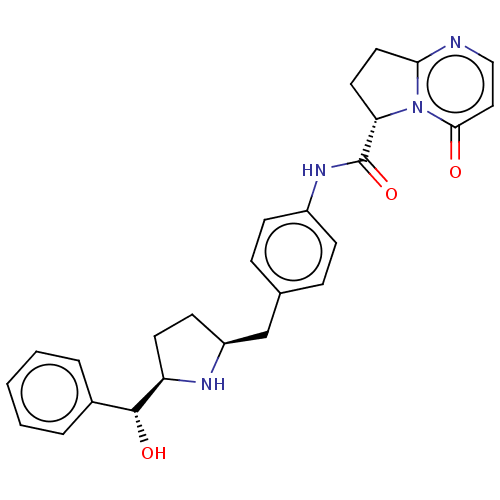 (KRP-114V | MK-4618 | Vibegron) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50092645 ((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes assessed as dextraomethorphan O-demethylation | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50146155 (CHEMBL3764774) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-diltiazem from human Cav1.2 channel | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50338821 (2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG channel | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50146154 (KRP-114V | MK-4618 | Vibegron) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50448787 (CHEMBL3128178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50448789 (CHEMBL3128188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50146161 (CHEMBL3763998) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50146156 (CHEMBL3764088) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50146157 (CHEMBL3764950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50146158 (CHEMBL3763594) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50146159 (CHEMBL3764592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50146160 (CHEMBL3765335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50146161 (CHEMBL3763998) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50146162 (CHEMBL3763934) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50146154 (KRP-114V | MK-4618 | Vibegron) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50146160 (CHEMBL3765335) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50146159 (CHEMBL3764592) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50146158 (CHEMBL3763594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50146157 (CHEMBL3764950) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50146162 (CHEMBL3763934) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50338821 (2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50146156 (CHEMBL3764088) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50146155 (CHEMBL3764774) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50448789 (CHEMBL3128188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50448787 (CHEMBL3128178) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50338821 (2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method | J Med Chem 59: 609-23 (2016) Article DOI: 10.1021/acs.jmedchem.5b01372 BindingDB Entry DOI: 10.7270/Q2M047B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 142 total ) | Next | Last >> |