 Found 132 hits with Last Name = 'frick' and Initial = 'k'
Found 132 hits with Last Name = 'frick' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50048864

(CHEMBL3310505)Show SMILES [H][C@@]12CC(=O)N[C@H](C(C)C)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O2 |r,t:20| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17+,19+,20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50048864

(CHEMBL3310505)Show SMILES [H][C@@]12CC(=O)N[C@H](C(C)C)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O2 |r,t:20| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17+,19+,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-tagged estrogen receptor alpha ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay |
J Med Chem 61: 4720-4738 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01601
BindingDB Entry DOI: 10.7270/Q2TH8R3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-tagged estrogen receptor beta ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay |
J Med Chem 61: 4720-4738 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01601
BindingDB Entry DOI: 10.7270/Q2TH8R3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50048864

(CHEMBL3310505)Show SMILES [H][C@@]12CC(=O)N[C@H](C(C)C)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O2 |r,t:20| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17+,19+,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC2 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50048864

(CHEMBL3310505)Show SMILES [H][C@@]12CC(=O)N[C@H](C(C)C)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O2 |r,t:20| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17+,19+,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC8 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50048864

(CHEMBL3310505)Show SMILES [H][C@@]12CC(=O)N[C@H](C(C)C)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O2 |r,t:20| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17+,19+,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC6 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50517935
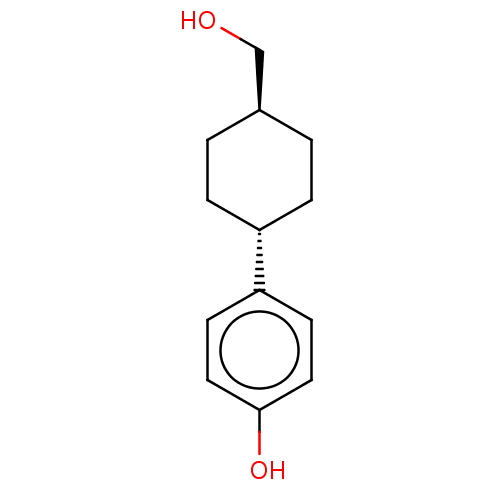
(CHEMBL4526434)Show SMILES OC[C@H]1CC[C@@H](CC1)c1ccc(O)cc1 |r,wU:5.8,wD:2.1,(30.01,-26.44,;29.24,-27.77,;27.7,-27.77,;26.93,-26.43,;25.39,-26.43,;24.62,-27.76,;25.39,-29.1,;26.93,-29.1,;23.08,-27.76,;22.32,-26.42,;20.78,-26.42,;20.01,-27.76,;18.47,-27.76,;20.79,-29.09,;22.32,-29.09,)| Show InChI InChI=1S/C13H18O2/c14-9-10-1-3-11(4-2-10)12-5-7-13(15)8-6-12/h5-8,10-11,14-15H,1-4,9H2/t10-,11- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-tagged estrogen receptor beta ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay |
J Med Chem 61: 4720-4738 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01601
BindingDB Entry DOI: 10.7270/Q2TH8R3Q |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517935

(CHEMBL4526434)Show SMILES OC[C@H]1CC[C@@H](CC1)c1ccc(O)cc1 |r,wU:5.8,wD:2.1,(30.01,-26.44,;29.24,-27.77,;27.7,-27.77,;26.93,-26.43,;25.39,-26.43,;24.62,-27.76,;25.39,-29.1,;26.93,-29.1,;23.08,-27.76,;22.32,-26.42,;20.78,-26.42,;20.01,-27.76,;18.47,-27.76,;20.79,-29.09,;22.32,-29.09,)| Show InChI InChI=1S/C13H18O2/c14-9-10-1-3-11(4-2-10)12-5-7-13(15)8-6-12/h5-8,10-11,14-15H,1-4,9H2/t10-,11- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-tagged estrogen receptor alpha ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay |
J Med Chem 61: 4720-4738 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01601
BindingDB Entry DOI: 10.7270/Q2TH8R3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50585748

(CHEMBL5086240)Show SMILES CC(C)(C)OC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)OC(C)(C)C | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50585748

(CHEMBL5086240)Show SMILES CC(C)(C)OC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)OC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585748

(CHEMBL5086240)Show SMILES CC(C)(C)OC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)OC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 preincubated for 20 mins with DTT followed by enzyme addition and measured after 10 mins by Glo-lum... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585748

(CHEMBL5086240)Show SMILES CC(C)(C)OC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)OC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC2 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585743

(CHEMBL5087342)Show SMILES CCCC[C@@H](NC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)N[C@H](CCCC)C(O)=O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 preincubated for 20 mins with DTT followed by enzyme addition and measured after 10 mins by Glo-lum... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50585748

(CHEMBL5086240)Show SMILES CC(C)(C)OC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)OC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC8 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50585748

(CHEMBL5086240)Show SMILES CC(C)(C)OC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)OC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC6 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585744
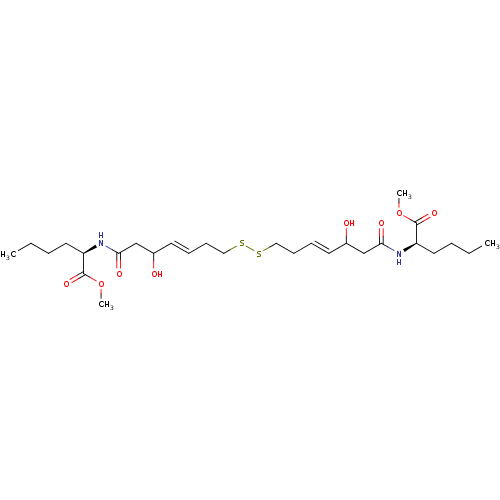
(CHEMBL5081652)Show SMILES CCCC[C@@H](NC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)N[C@H](CCCC)C(=O)OC)C(=O)OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 preincubated for 20 mins with DTT followed by enzyme addition and measured after 10 mins by Glo-lum... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585746
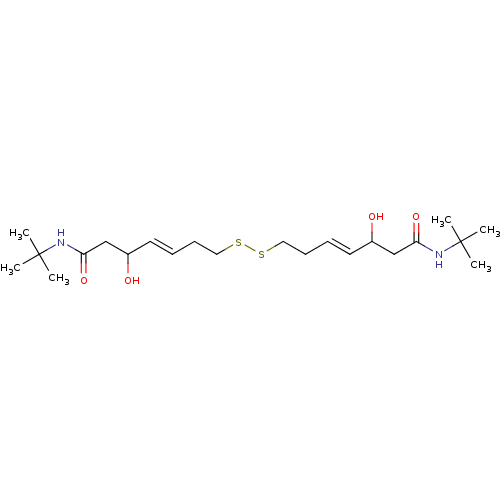
(CHEMBL5085306)Show SMILES CC(C)(C)NC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)NC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 preincubated for 20 mins with DTT followed by enzyme addition and measured after 10 mins by Glo-lum... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585745

(CHEMBL5084611)Show SMILES OC(CC(=O)Nc1ccccc1)\C=C\CCSSCC\C=C\C(O)CC(=O)Nc1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 preincubated for 20 mins with DTT followed by enzyme addition and measured after 10 mins by Glo-lum... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585747
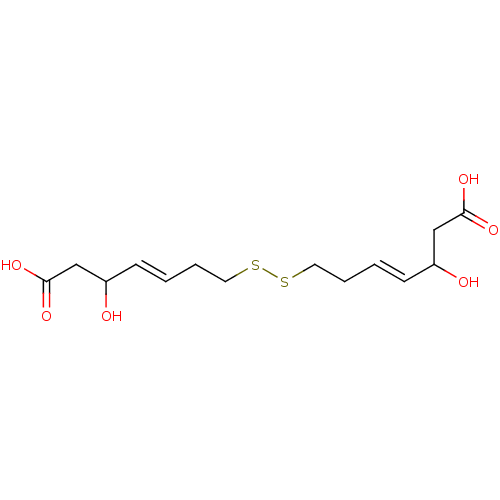
(CHEMBL5074871) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 preincubated for 20 mins with DTT followed by enzyme addition and measured after 10 mins by Glo-lum... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585746

(CHEMBL5085306)Show SMILES CC(C)(C)NC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)NC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 incubated for 10 mins by Glo-luminescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585748

(CHEMBL5086240)Show SMILES CC(C)(C)OC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)OC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 incubated for 10 mins by Glo-luminescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585745

(CHEMBL5084611)Show SMILES OC(CC(=O)Nc1ccccc1)\C=C\CCSSCC\C=C\C(O)CC(=O)Nc1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 incubated for 10 mins by Glo-luminescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585747

(CHEMBL5074871) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 incubated for 10 mins by Glo-luminescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585743

(CHEMBL5087342)Show SMILES CCCC[C@@H](NC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)N[C@H](CCCC)C(O)=O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 incubated for 10 mins by Glo-luminescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50585744

(CHEMBL5081652)Show SMILES CCCC[C@@H](NC(=O)CC(O)\C=C\CCSSCC\C=C\C(O)CC(=O)N[C@H](CCCC)C(=O)OC)C(=O)OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length HDAC2 incubated for 10 mins by Glo-luminescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01928
BindingDB Entry DOI: 10.7270/Q2H41WB8 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185697

(CHEMBL3823077)Show SMILES CS(=O)(=O)NC(=O)c1ccc(cc1)[C@@H]1C[C@H]1c1ccc(OCc2c(noc2C2CC2)-c2c(Cl)cccc2Cl)cc1Cl |r,wU:15.17,wD:13.13,(13.72,-.73,;12.5,-.93,;13.28,-1.88,;12.94,.22,;10.98,-1.18,;10,.02,;10.44,1.17,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.73,.9,;-7.76,2.04,;-6.99,3.37,;-5.48,3.05,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C30H25Cl3N2O5S/c1-41(37,38)35-30(36)18-9-5-16(6-10-18)21-14-22(21)20-12-11-19(13-26(20)33)39-15-23-28(34-40-29(23)17-7-8-17)27-24(31)3-2-4-25(27)32/h2-6,9-13,17,21-22H,7-8,14-15H2,1H3,(H,35,36)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185698

(CHEMBL3823213)Show SMILES CS(=O)(=O)NC(=O)c1ccc(cc1)C1CC1c1ccc(OCc2c(noc2C2CC2)-c2c(Cl)cccc2Cl)cc1Cl |(13.72,-.73,;12.5,-.93,;13.28,-1.88,;12.94,.22,;10.98,-1.18,;10,.02,;10.44,1.17,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.73,.9,;-7.76,2.04,;-6.99,3.37,;-5.48,3.05,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C30H25Cl3N2O5S/c1-41(37,38)35-30(36)18-9-5-16(6-10-18)21-14-22(21)20-12-11-19(13-26(20)33)39-15-23-28(34-40-29(23)17-7-8-17)27-24(31)3-2-4-25(27)32/h2-6,9-13,17,21-22H,7-8,14-15H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185699

(CHEMBL3824337)Show SMILES Cn1nc(C(O)=O)c2ccc(cc12)C1CC1c1ccc(OCc2c(noc2C2CC2)-c2c(Cl)cccc2Cl)cc1Cl |(2.14,2.41,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;1.42,-3.62,;3.45,-2.94,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;.3,.77,;-3.71,1.53,;-5.17,1.49,;-4.4,2.82,;-4.38,4.36,;-5.92,4.59,;-6.46,6.03,;-5.49,7.23,;-6.04,8.67,;-7.56,8.91,;-8.1,10.35,;-9.58,10.74,;-9.66,12.28,;-8.21,12.82,;-7.25,11.62,;-5.71,11.68,;-4.38,11.09,;-4.59,12.62,;-10.78,9.77,;-12.17,10.44,;-12.26,11.66,;-13.44,9.56,;-13.32,8.03,;-11.93,7.36,;-10.66,8.24,;-9.54,7.71,;-3.97,6.98,;-3.42,5.54,;-2.21,5.34,)| Show InChI InChI=1S/C31H24Cl3N3O4/c1-37-26-11-16(7-9-19(26)29(35-37)31(38)39)20-13-21(20)18-10-8-17(12-25(18)34)40-14-22-28(36-41-30(22)15-5-6-15)27-23(32)3-2-4-24(27)33/h2-4,7-12,15,20-21H,5-6,13-14H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185699

(CHEMBL3824337)Show SMILES Cn1nc(C(O)=O)c2ccc(cc12)C1CC1c1ccc(OCc2c(noc2C2CC2)-c2c(Cl)cccc2Cl)cc1Cl |(2.14,2.41,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;1.42,-3.62,;3.45,-2.94,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;.3,.77,;-3.71,1.53,;-5.17,1.49,;-4.4,2.82,;-4.38,4.36,;-5.92,4.59,;-6.46,6.03,;-5.49,7.23,;-6.04,8.67,;-7.56,8.91,;-8.1,10.35,;-9.58,10.74,;-9.66,12.28,;-8.21,12.82,;-7.25,11.62,;-5.71,11.68,;-4.38,11.09,;-4.59,12.62,;-10.78,9.77,;-12.17,10.44,;-12.26,11.66,;-13.44,9.56,;-13.32,8.03,;-11.93,7.36,;-10.66,8.24,;-9.54,7.71,;-3.97,6.98,;-3.42,5.54,;-2.21,5.34,)| Show InChI InChI=1S/C31H24Cl3N3O4/c1-37-26-11-16(7-9-19(26)29(35-37)31(38)39)20-13-21(20)18-10-8-17(12-25(18)34)40-14-22-28(36-41-30(22)15-5-6-15)27-23(32)3-2-4-24(27)33/h2-4,7-12,15,20-21H,5-6,13-14H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185710
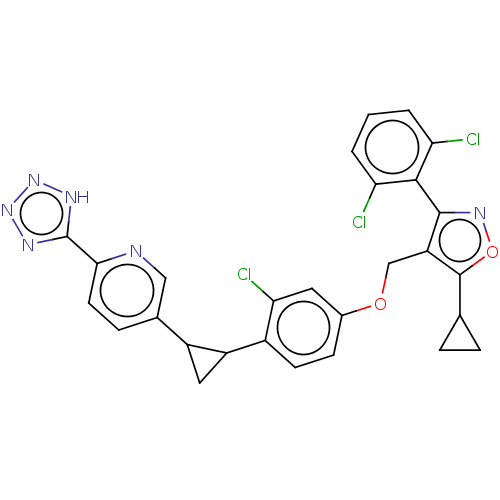
(CHEMBL3823312)Show SMILES Clc1cccc(Cl)c1-c1noc(C2CC2)c1COc1ccc(C2CC2c2ccc(nc2)-c2nnn[nH]2)c(Cl)c1 |(-9.39,-.09,;-8.54,-.98,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-7.04,-.61,;-6.73,.9,;-7.76,2.04,;-6.99,3.37,;-5.48,3.05,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;-5.34,1.53,;-4,.77,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;2.67,-1.54,;3.35,-2.83,;4.13,-1.5,;5.45,-.71,;5.99,.71,;7.51,.96,;8.48,-.23,;7.94,-1.67,;6.42,-1.92,;10,.02,;10.68,1.39,;12.2,1.16,;12.44,-.36,;11.07,-1.07,;1.33,.77,;2.4,1.39,;,1.54,)| Show InChI InChI=1S/C28H21Cl3N6O2/c29-21-2-1-3-22(30)25(21)26-20(27(39-35-26)14-4-5-14)13-38-16-7-8-17(23(31)10-16)19-11-18(19)15-6-9-24(32-12-15)28-33-36-37-34-28/h1-3,6-10,12,14,18-19H,4-5,11,13H2,(H,33,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 111 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185725
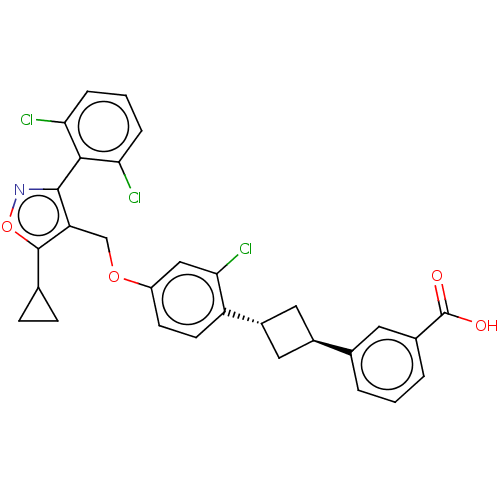
(CHEMBL3822859)Show SMILES OC(=O)c1cccc(c1)[C@H]1C[C@@H](C1)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:9.9,wD:11.14,(10.79,-2.28,;9.63,-1.88,;9.4,-.67,;8.46,-2.88,;8.74,-4.39,;7.57,-5.39,;6.12,-4.88,;5.84,-3.38,;7.01,-2.37,;4.51,-2.61,;3.02,-3,;2.67,-1.54,;4.11,-1.12,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C30H24Cl3NO4/c31-24-5-2-6-25(32)27(24)28-23(29(38-34-28)16-7-8-16)15-37-21-9-10-22(26(33)14-21)20-12-19(13-20)17-3-1-4-18(11-17)30(35)36/h1-6,9-11,14,16,19-20H,7-8,12-13,15H2,(H,35,36)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185697

(CHEMBL3823077)Show SMILES CS(=O)(=O)NC(=O)c1ccc(cc1)[C@@H]1C[C@H]1c1ccc(OCc2c(noc2C2CC2)-c2c(Cl)cccc2Cl)cc1Cl |r,wU:15.17,wD:13.13,(13.72,-.73,;12.5,-.93,;13.28,-1.88,;12.94,.22,;10.98,-1.18,;10,.02,;10.44,1.17,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.73,.9,;-7.76,2.04,;-6.99,3.37,;-5.48,3.05,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C30H25Cl3N2O5S/c1-41(37,38)35-30(36)18-9-5-16(6-10-18)21-14-22(21)20-12-11-19(13-26(20)33)39-15-23-28(34-40-29(23)17-7-8-17)27-24(31)3-2-4-25(27)32/h2-6,9-13,17,21-22H,7-8,14-15H2,1H3,(H,35,36)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185721
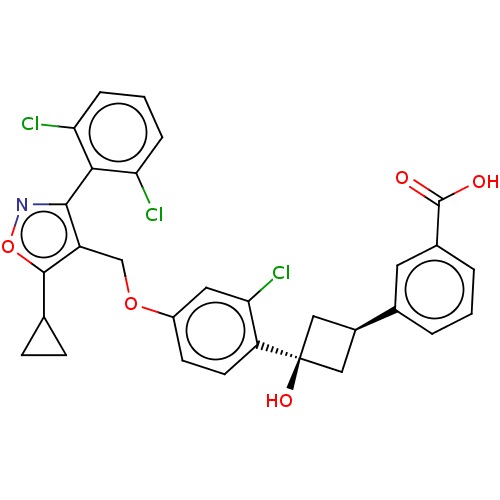
(CHEMBL3822901)Show SMILES OC(=O)c1cccc(c1)[C@H]1C[C@@](O)(C1)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:9.9,wD:11.15,(7.33,5.1,;6.4,4.3,;5.24,4.71,;6.68,2.79,;8.14,2.27,;8.42,.76,;7.25,-.24,;5.81,.27,;5.51,1.78,;4.47,-.5,;4.07,-1.99,;2.67,-1.54,;2.67,-2.77,;2.99,-.1,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C30H24Cl3NO5/c31-23-5-2-6-24(32)26(23)27-21(28(39-34-27)16-7-8-16)15-38-20-9-10-22(25(33)12-20)30(37)13-19(14-30)17-3-1-4-18(11-17)29(35)36/h1-6,9-12,16,19,37H,7-8,13-15H2,(H,35,36)/t19-,30+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at GST-tagged FXR LBD (187 to 472 residues) (unknown origin) assessed as FXR interaction with b-CPSSHSSLTERHKILHRLLQEGSPS-COOH by FR... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185706
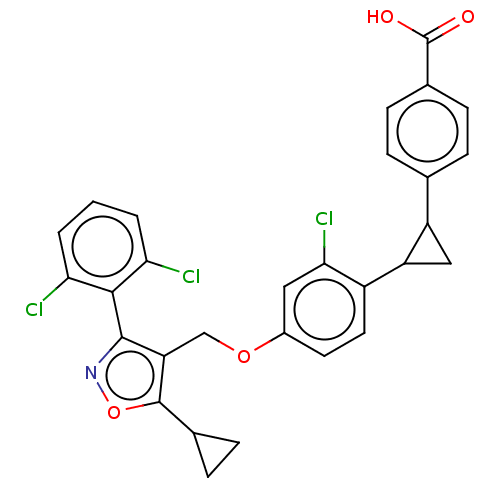
(CHEMBL3822803)Show SMILES OC(=O)c1ccc(cc1)C1CC1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |(10.44,1.17,;10,.02,;10.78,-.94,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C29H22Cl3NO4/c30-23-2-1-3-24(31)26(23)27-22(28(37-33-27)16-6-7-16)14-36-18-10-11-19(25(32)12-18)21-13-20(21)15-4-8-17(9-5-15)29(34)35/h1-5,8-12,16,20-21H,6-7,13-14H2,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185707

(CHEMBL3822773)Show SMILES OC(=O)c1ccc(cc1)[C@H]1C[C@@H]1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:9.9,wD:11.13,(10.44,1.17,;10,.02,;10.78,-.94,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C29H22Cl3NO4/c30-23-2-1-3-24(31)26(23)27-22(28(37-33-27)16-6-7-16)14-36-18-10-11-19(25(32)12-18)21-13-20(21)15-4-8-17(9-5-15)29(34)35/h1-5,8-12,16,20-21H,6-7,13-14H2,(H,34,35)/t20-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 186 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185708

(CHEMBL3822464)Show SMILES OC(=O)c1ccc(cc1)[C@@H]1C[C@H]1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:11.13,wD:9.9,(10.44,1.17,;10,.02,;10.78,-.94,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C29H22Cl3NO4/c30-23-2-1-3-24(31)26(23)27-22(28(37-33-27)16-6-7-16)14-36-18-10-11-19(25(32)12-18)21-13-20(21)15-4-8-17(9-5-15)29(34)35/h1-5,8-12,16,20-21H,6-7,13-14H2,(H,34,35)/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185709
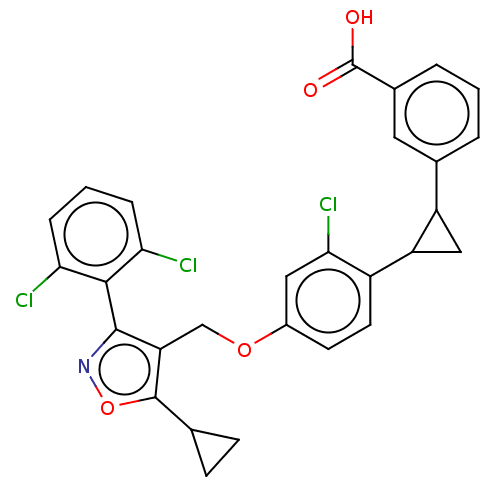
(CHEMBL3822597)Show SMILES OC(=O)c1cccc(c1)C1CC1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |(10.13,-2.67,;8.91,-2.86,;8.48,-4.02,;7.94,-1.67,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C29H22Cl3NO4/c30-23-5-2-6-24(31)26(23)27-22(28(37-33-27)15-7-8-15)14-36-18-9-10-19(25(32)12-18)21-13-20(21)16-3-1-4-17(11-16)29(34)35/h1-6,9-12,15,20-21H,7-8,13-14H2,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185705

(CHEMBL3824077)Show SMILES OC(=O)c1cccc(c1)[C@H]1C[C@@H]1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:9.9,wD:11.13,(10.13,-2.67,;8.91,-2.86,;8.48,-4.02,;7.94,-1.67,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C29H22Cl3NO4/c30-23-5-2-6-24(31)26(23)27-22(28(37-33-27)15-7-8-15)14-36-18-9-10-19(25(32)12-18)21-13-20(21)16-3-1-4-17(11-16)29(34)35/h1-6,9-12,15,20-21H,7-8,13-14H2,(H,34,35)/t20-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185704

(CHEMBL3824162)Show SMILES OC(=O)c1cccc(c1)[C@@H]1C[C@H]1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:11.13,wD:9.9,(10.13,-2.67,;8.91,-2.86,;8.48,-4.02,;7.94,-1.67,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C29H22Cl3NO4/c30-23-5-2-6-24(31)26(23)27-22(28(37-33-27)15-7-8-15)14-36-18-9-10-19(25(32)12-18)21-13-20(21)16-3-1-4-17(11-16)29(34)35/h1-6,9-12,15,20-21H,7-8,13-14H2,(H,34,35)/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185703
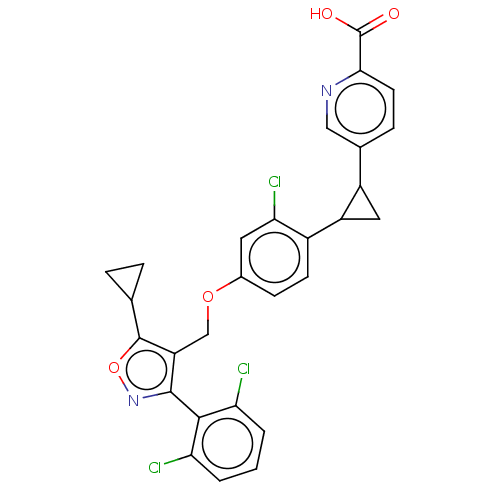
(CHEMBL3823070)Show SMILES OC(=O)c1ccc(cn1)C1CC1c1ccc(OCc2c(noc2C2CC2)-c2c(Cl)cccc2Cl)cc1Cl |(10.44,1.17,;10,.02,;10.78,-.94,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.73,.9,;-7.76,2.04,;-6.99,3.37,;-5.48,3.05,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C28H21Cl3N2O4/c29-21-2-1-3-22(30)25(21)26-20(27(37-33-26)14-4-5-14)13-36-16-7-8-17(23(31)10-16)19-11-18(19)15-6-9-24(28(34)35)32-12-15/h1-3,6-10,12,14,18-19H,4-5,11,13H2,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 57 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185702

(CHEMBL3824163)Show SMILES OC(=O)c1cncc(c1)C1CC1c1ccc(OCc2c(noc2C2CC2)-c2c(Cl)cccc2Cl)cc1Cl |(7.28,3.36,;8.06,2.4,;9.27,2.6,;7.51,.96,;8.48,-.23,;7.94,-1.67,;6.42,-1.92,;5.45,-.71,;5.99,.71,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.73,.9,;-7.76,2.04,;-6.99,3.37,;-5.48,3.05,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C28H21Cl3N2O4/c29-22-2-1-3-23(30)25(22)26-21(27(37-33-26)14-4-5-14)13-36-17-6-7-18(24(31)9-17)20-10-19(20)15-8-16(28(34)35)12-32-11-15/h1-3,6-9,11-12,14,19-20H,4-5,10,13H2,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185701

(CHEMBL3822798)Show SMILES CC(C)n1nc(cc1C1CC1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl)C(O)=O |(6.11,3.04,;5.44,2.01,;4.21,2.08,;6.13,.64,;7.65,.38,;7.88,-1.14,;6.5,-1.83,;5.45,-.71,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,;9.24,-1.85,;9.3,-3.08,;10.28,-1.19,)| Show InChI InChI=1S/C29H26Cl3N3O4/c1-14(2)35-25(12-24(33-35)29(36)37)19-11-18(19)17-9-8-16(10-23(17)32)38-13-20-27(34-39-28(20)15-6-7-15)26-21(30)4-3-5-22(26)31/h3-5,8-10,12,14-15,18-19H,6-7,11,13H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185700
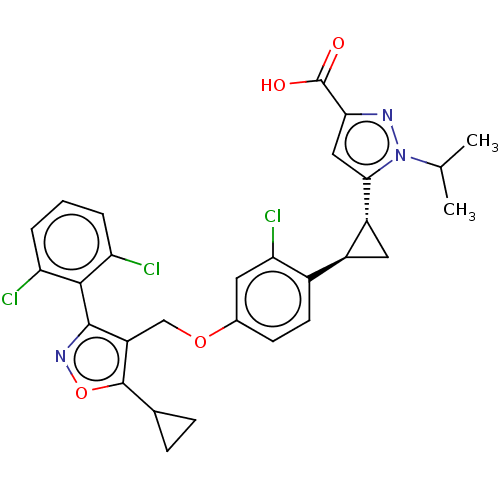
(CHEMBL3822755)Show SMILES CC(C)n1nc(cc1[C@@H]1C[C@H]1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl)C(O)=O |r,wU:10.12,wD:8.8,(6.11,3.04,;5.44,2.01,;4.21,2.08,;6.13,.64,;7.65,.38,;7.88,-1.14,;6.5,-1.83,;5.45,-.71,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,;9.24,-1.85,;9.3,-3.08,;10.28,-1.19,)| Show InChI InChI=1S/C29H26Cl3N3O4/c1-14(2)35-25(12-24(33-35)29(36)37)19-11-18(19)17-9-8-16(10-23(17)32)38-13-20-27(34-39-28(20)15-6-7-15)26-21(30)4-3-5-22(26)31/h3-5,8-10,12,14-15,18-19H,6-7,11,13H2,1-2H3,(H,36,37)/t18-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185724
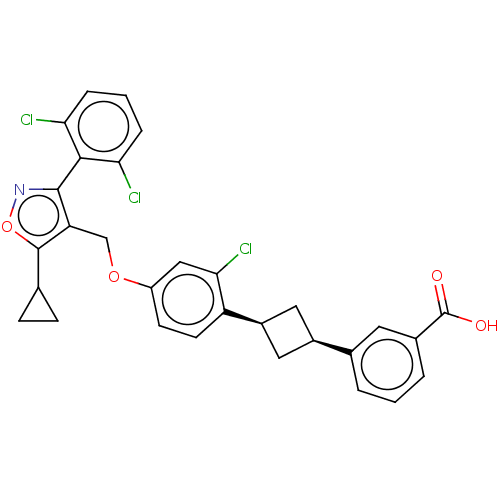
(CHEMBL3823491)Show SMILES OC(=O)c1cccc(c1)[C@H]1C[C@H](C1)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:11.14,9.9,(10.79,-2.28,;9.63,-1.88,;9.4,-.67,;8.46,-2.88,;8.74,-4.39,;7.57,-5.39,;6.12,-4.88,;5.84,-3.38,;7.01,-2.37,;4.51,-2.61,;3.02,-3,;2.67,-1.54,;4.11,-1.12,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C30H24Cl3NO4/c31-24-5-2-6-25(32)27(24)28-23(29(38-34-28)16-7-8-16)15-37-21-9-10-22(26(33)14-21)20-12-19(13-20)17-3-1-4-18(11-17)30(35)36/h1-6,9-11,14,16,19-20H,7-8,12-13,15H2,(H,35,36)/t19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 92 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185698

(CHEMBL3823213)Show SMILES CS(=O)(=O)NC(=O)c1ccc(cc1)C1CC1c1ccc(OCc2c(noc2C2CC2)-c2c(Cl)cccc2Cl)cc1Cl |(13.72,-.73,;12.5,-.93,;13.28,-1.88,;12.94,.22,;10.98,-1.18,;10,.02,;10.44,1.17,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.73,.9,;-7.76,2.04,;-6.99,3.37,;-5.48,3.05,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C30H25Cl3N2O5S/c1-41(37,38)35-30(36)18-9-5-16(6-10-18)21-14-22(21)20-12-11-19(13-26(20)33)39-15-23-28(34-40-29(23)17-7-8-17)27-24(31)3-2-4-25(27)32/h2-6,9-13,17,21-22H,7-8,14-15H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185718

(CHEMBL3823637)Show SMILES CO[C@@]1(C[C@@H](C1)c1cccc(c1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:4.6,wD:2.16,(3.73,-3.7,;2.66,-3.08,;2.67,-1.54,;4.07,-1.99,;4.47,-.51,;2.98,-.1,;5.81,.26,;7.25,-.25,;8.42,.75,;8.14,2.27,;6.69,2.78,;5.51,1.78,;6.4,4.3,;7.34,5.1,;5.24,4.7,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C31H26Cl3NO5/c1-38-31(14-20(15-31)18-4-2-5-19(12-18)30(36)37)23-11-10-21(13-26(23)34)39-16-22-28(35-40-29(22)17-8-9-17)27-24(32)6-3-7-25(27)33/h2-7,10-13,17,20H,8-9,14-16H2,1H3,(H,36,37)/t20-,31+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 102 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185710

(CHEMBL3823312)Show SMILES Clc1cccc(Cl)c1-c1noc(C2CC2)c1COc1ccc(C2CC2c2ccc(nc2)-c2nnn[nH]2)c(Cl)c1 |(-9.39,-.09,;-8.54,-.98,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-7.04,-.61,;-6.73,.9,;-7.76,2.04,;-6.99,3.37,;-5.48,3.05,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;-5.34,1.53,;-4,.77,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;2.67,-1.54,;3.35,-2.83,;4.13,-1.5,;5.45,-.71,;5.99,.71,;7.51,.96,;8.48,-.23,;7.94,-1.67,;6.42,-1.92,;10,.02,;10.68,1.39,;12.2,1.16,;12.44,-.36,;11.07,-1.07,;1.33,.77,;2.4,1.39,;,1.54,)| Show InChI InChI=1S/C28H21Cl3N6O2/c29-21-2-1-3-22(30)25(21)26-20(27(39-35-26)14-4-5-14)13-38-16-7-8-17(23(31)10-16)19-11-18(19)15-6-9-24(32-12-15)28-33-36-37-34-28/h1-3,6-10,12,14,18-19H,4-5,11,13H2,(H,33,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 111 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at C-terminal Gal4-tagged human FXR (187 to 472 residues) expressed in HEK-293 cells co-expressing pFRluc by mammalian one hybrid as... |
Bioorg Med Chem Lett 26: 3746-53 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.070
BindingDB Entry DOI: 10.7270/Q2C24ZB9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data