

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305271 (5-[2-(4-Fluorophenyl)ethyl]-2,8-dimethyl-2,3,4,5-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305268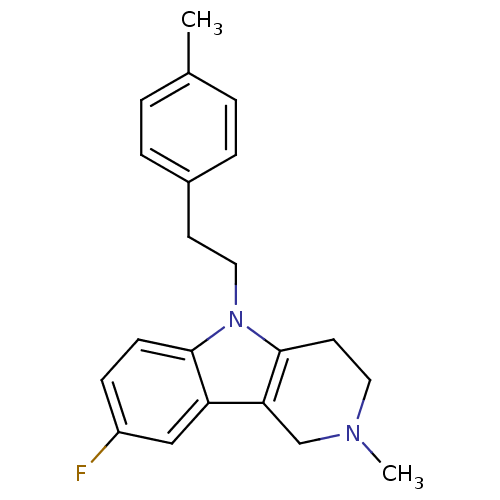 (8-Fluoro-2-methyl-5-(2-p-tolylethyl)-2,3,4,5-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305266 (8-Fluoro-5-[2-(4-methoxyphenyl)ethyl]-2-methyl-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305260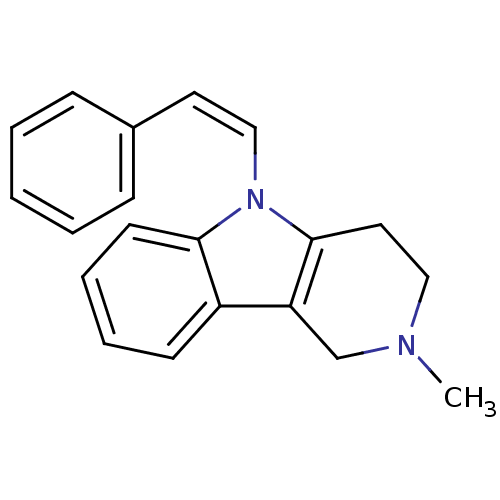 (2-Methyl-5-((Z)-styryl)-2,3,4,5-tetrahydro-1H-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305267 (8-Fluoro-2-methyl-5-[2-(4-trifluoromethylphenyl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305262 (8-Fluoro-2-methyl-5-phenethyl-2,3,4,5-tetrahydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305263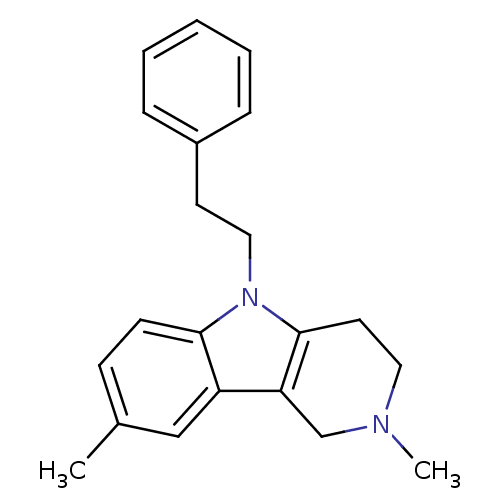 (2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305269 (5-[2-(4-Methoxyphenyl)ethyl]-2,8-dimethyl-2,3,4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305265 (2-Methyl-5-phenethyl-8-trifluoromethyl-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305258 (2,8-Dimethyl-5-((Z)-styryl)-2,3,4,5-tetrahydro-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50305258 (2,8-Dimethyl-5-((Z)-styryl)-2,3,4,5-tetrahydro-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5HT6 receptor expressed in HEK-293 cells assessed as inhibition of serotonin-induced increase in intracellul... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305270 (2,8-Dimethyl-5-[2-(4-trifluoromethylphenyl)ethyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346044 (2-methyl-5-(2-(pyridin-3-yl)ethyl)-2,3,4,5-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305264 (8-Methoxy-2-methyl-5-phenethyl-2,3,4,5-tetrahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346052 (8-fluoro-2-methyl-5-(2-(6-methylpyridin-3-yl)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305272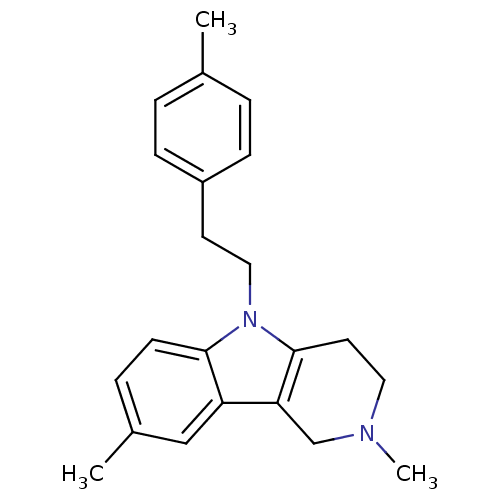 (2,8-Dimethyl-5-(2-p-tolylethyl)-2,3,4,5-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346050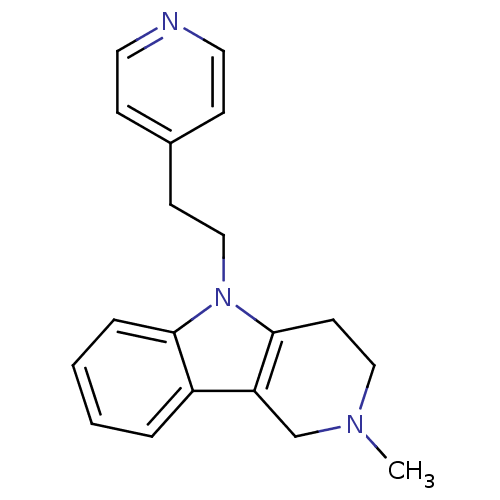 (2-methyl-5-(2-(pyridin-4-yl)ethyl)-2,3,4,5-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346058 (8-fluoro-2-methyl-5-(2-(pyridin-4-yl)ethyl)-2,3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346057 (8-bromo-2-methyl-5-(2-(pyridin-2-yl)ethyl)-2,3,4,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305262 (8-Fluoro-2-methyl-5-phenethyl-2,3,4,5-tetrahydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as reduction of histamine-induced intracellular calcium spike at phase 2... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305263 (2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as reduction of histamine-induced intracellular calcium spike at phase 2... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305258 (2,8-Dimethyl-5-((Z)-styryl)-2,3,4,5-tetrahydro-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as reduction of histamine-induced intracellular calcium spike at phase 2... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305261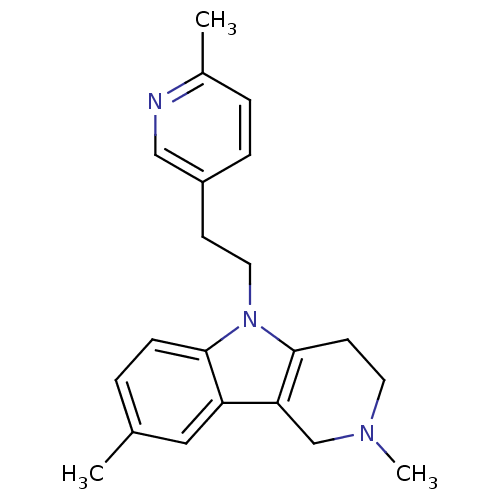 (CHEMBL589390 | Dimebolin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50305263 (2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5HT6 receptor expressed in HEK-293 cells assessed as inhibition of serotonin-induced increase in intracellul... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305261 (CHEMBL589390 | Dimebolin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346059 (2,8-dimethyl-5-(2-(pyridin-4-yl)ethyl)-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50305255 (25-[(Z)-2-(4-Fluorophenyl)vinyl]-2,8-dimethyl-2,3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5HT6 receptor expressed in HEK-293 cells assessed as inhibition of serotonin-induced increase in intracellul... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305260 (2-Methyl-5-((Z)-styryl)-2,3,4,5-tetrahydro-1H-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as reduction of histamine-induced intracellular calcium spike at phase 2... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346045 (2-methyl-5-(2-(pyridin-4-yl)ethyl)-8-(trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50305272 (2,8-Dimethyl-5-(2-p-tolylethyl)-2,3,4,5-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 276 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5HT6 receptor expressed in HEK-293 cells assessed as inhibition of serotonin-induced increase in intracellul... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346049 (2,8-dimethyl-5-(2-pyrazine-2-ylethyl)-gamma-carbol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50305259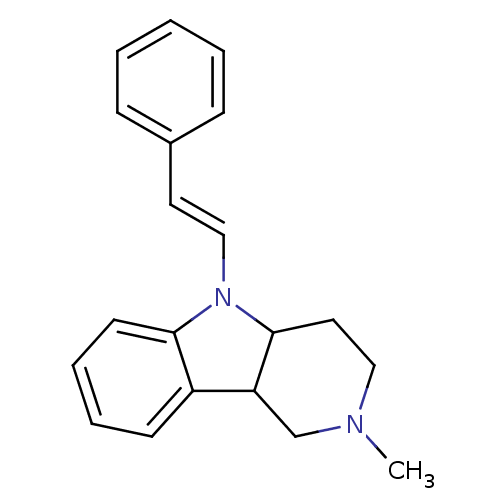 (2-Methyl-5-((E)-styryl)-2,3,4,5-tetrahydro-1H-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 345 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in human SK-N-SH cells assessed as histamine-induced maximum intracellular calcium spike at phase 1 trea... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50346059 (2,8-dimethyl-5-(2-(pyridin-4-yl)ethyl)-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5-HT6 receptor expressed in HEK293 cells assessed as inhibition of serotonin-induced intracellular cAMP accu... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346060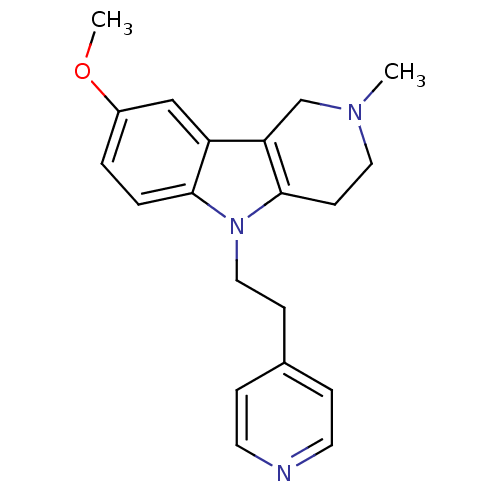 (8-methoxy-2-methyl-5-(2-(pyridin-4-yl)ethyl)-2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346044 (2-methyl-5-(2-(pyridin-3-yl)ethyl)-2,3,4,5-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium flow during phase-II compound... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50346061 (2,8-dimethyl-5-(2-(pyridin-3-yl)ethyl)-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5-HT6 receptor expressed in HEK293 cells assessed as inhibition of serotonin-induced intracellular cAMP accu... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346058 (8-fluoro-2-methyl-5-(2-(pyridin-4-yl)ethyl)-2,3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium flow during phase-II compound... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346061 (2,8-dimethyl-5-(2-(pyridin-3-yl)ethyl)-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346050 (2-methyl-5-(2-(pyridin-4-yl)ethyl)-2,3,4,5-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium flow during phase-II compound... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346051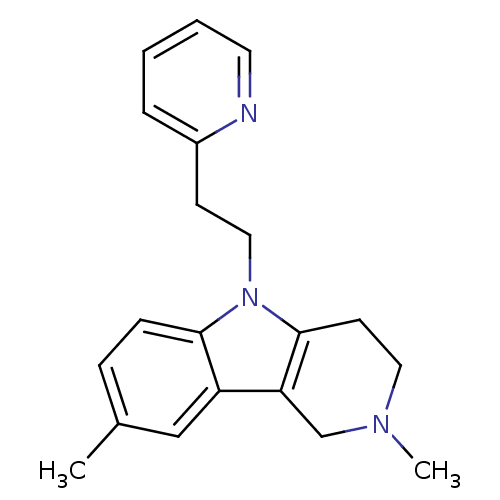 (2,8-dimethyl-5-(2-(pyridin-2-yl)ethyl)-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50305253 (5-[(Z)-2-(4-Methoxyphenyl)vinyl]-2,8-dimethyl-2,3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5HT6 receptor expressed in HEK-293 cells assessed as inhibition of serotonin-induced increase in intracellul... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346062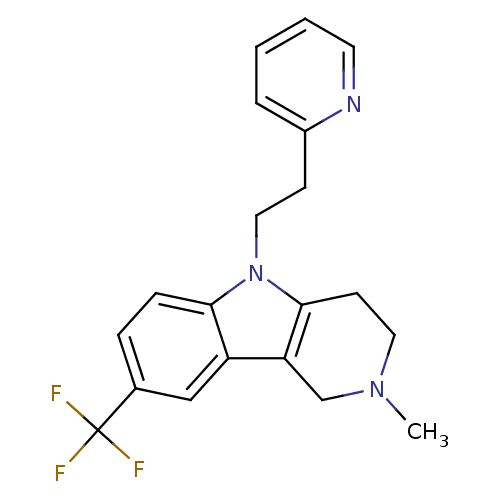 (2-methyl-5-(2-(pyridin-2-yl)ethyl)-8-(trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346063 (8-fluoro-2-methyl-5-(2-(pyridin-2-yl)ethyl)-2,3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium flow during phase-II compound... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346056 (8-fluoro-2-methyl-5-(2-(pyridin-3-yl)ethyl)-2,3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium level increase during phase-1... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50305269 (5-[2-(4-Methoxyphenyl)ethyl]-2,8-dimethyl-2,3,4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 666 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5HT6 receptor expressed in HEK-293 cells assessed as inhibition of serotonin-induced increase in intracellul... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50305262 (8-Fluoro-2-methyl-5-phenethyl-2,3,4,5-tetrahydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 692 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5HT6 receptor expressed in HEK-293 cells assessed as inhibition of serotonin-induced increase in intracellul... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50346052 (8-fluoro-2-methyl-5-(2-(6-methylpyridin-3-yl)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H1 receptor in SK-N-SH cells assessed as inhibition of histamine-induced calcium flow during phase-II compound... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50346051 (2,8-dimethyl-5-(2-(pyridin-2-yl)ethyl)-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5-HT6 receptor expressed in HEK293 cells assessed as inhibition of serotonin-induced intracellular cAMP accu... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50305261 (CHEMBL589390 | Dimebolin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5-HT6 receptor expressed in HEK293 cells assessed as inhibition of serotonin-induced intracellular cAMP accu... | Bioorg Med Chem Lett 19: 3183-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.128 BindingDB Entry DOI: 10.7270/Q2FJ2H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50305256 (2,8-Dimethyl-5-((Z)-2-p-tolylvinyl)-2,3,4,5-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5HT6 receptor expressed in HEK-293 cells assessed as inhibition of serotonin-induced increase in intracellul... | Bioorg Med Chem Lett 20: 78-82 (2010) Article DOI: 10.1016/j.bmcl.2009.11.037 BindingDB Entry DOI: 10.7270/Q25Q4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 110 total ) | Next | Last >> |