 Found 62 hits with Last Name = 'fuchs' and Initial = 'je'
Found 62 hits with Last Name = 'fuchs' and Initial = 'je' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505841

(CHEMBL4532034 | US11174245, # I-018)Show SMILES COc1ccncc1-c1cc(ccn1)C(=O)\N=c1/[nH]c2ccccc2n1CC(C)(C)O Show InChI InChI=1S/C23H23N5O3/c1-23(2,30)14-28-19-7-5-4-6-17(19)26-22(28)27-21(29)15-8-11-25-18(12-15)16-13-24-10-9-20(16)31-3/h4-13,30H,14H2,1-3H3,(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of GST-fusion tagged EGFR L858R/T790M/C797S triple mutant (unknown origin) (696 to 1022 residues) expressed in insect cells assessed as de... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Mus musculus) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Mus musculus) | BDBM50505838
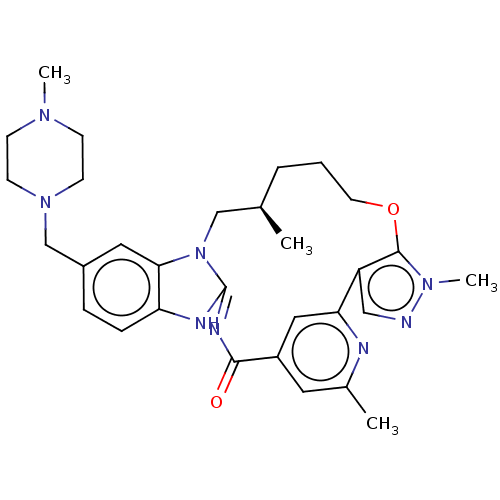
(CHEMBL4537790)Show SMILES C[C@@H]1CCCOc2c(cnn2C)-c2cc(cc(C)n2)C(=O)\N=C2/Nc3ccc(CN4CCN(C)CC4)cc3N2C1 |r,c:23| Show InChI InChI=1S/C30H38N8O2/c1-20-6-5-13-40-29-24(17-31-36(29)4)26-16-23(14-21(2)32-26)28(39)34-30-33-25-8-7-22(15-27(25)38(30)18-20)19-37-11-9-35(3)10-12-37/h7-8,14-17,20H,5-6,9-13,18-19H2,1-4H3,(H,33,34,39)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505836
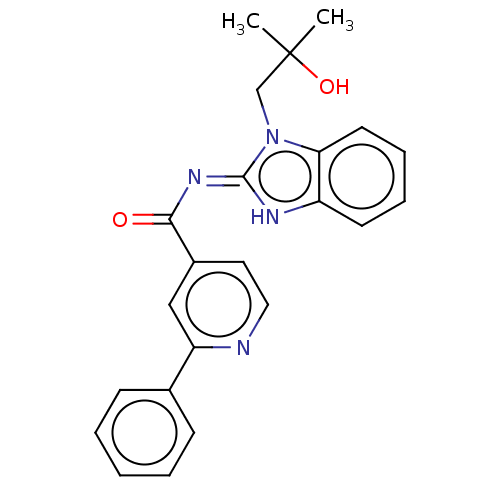
(CHEMBL4434788 | US11174245, # I-064)Show SMILES CC(C)(O)Cn1c2ccccc2[nH]\c1=N/C(=O)c1ccnc(c1)-c1ccccc1 Show InChI InChI=1S/C23H22N4O2/c1-23(2,29)15-27-20-11-7-6-10-18(20)25-22(27)26-21(28)17-12-13-24-19(14-17)16-8-4-3-5-9-16/h3-14,29H,15H2,1-2H3,(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of GST-fusion tagged EGFR L858R/T790M/C797S triple mutant (unknown origin) (696 to 1022 residues) expressed in insect cells assessed as de... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Mus musculus) | BDBM50505845

(CHEMBL4566193)Show SMILES Cn1ncc-2c1OCCCCCN1\C(Nc3ccccc13)=N\C(=O)c1cc(F)nc-2c1 |t:23| Show InChI InChI=1S/C22H21FN6O2/c1-28-21-15(13-24-28)17-11-14(12-19(23)25-17)20(30)27-22-26-16-7-3-4-8-18(16)29(22)9-5-2-6-10-31-21/h3-4,7-8,11-13H,2,5-6,9-10H2,1H3,(H,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505843

(CHEMBL4449589 | US11174245, # I-235)Show SMILES CC(C)(O)Cn1c2ccccc2[nH]\c1=N/C(=O)c1ccnc(c1)-c1cccnc1 Show InChI InChI=1S/C22H21N5O2/c1-22(2,29)14-27-19-8-4-3-7-17(19)25-21(27)26-20(28)15-9-11-24-18(12-15)16-6-5-10-23-13-16/h3-13,29H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of GST-fusion tagged EGFR L858R/T790M/C797S triple mutant (unknown origin) (696 to 1022 residues) expressed in insect cells assessed as de... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505839
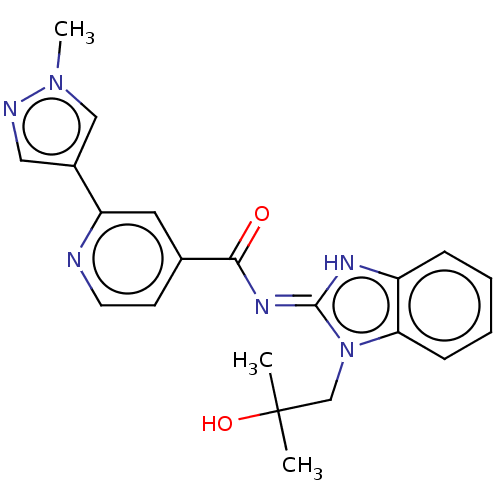
(CHEMBL4525935 | US11174245, # I-001)Show SMILES Cn1cc(cn1)-c1cc(ccn1)C(=O)\N=c1/[nH]c2ccccc2n1CC(C)(C)O Show InChI InChI=1S/C21H22N6O2/c1-21(2,29)13-27-18-7-5-4-6-16(18)24-20(27)25-19(28)14-8-9-22-17(10-14)15-11-23-26(3)12-15/h4-12,29H,13H2,1-3H3,(H,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 519 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of GST-fusion tagged EGFR L858R/T790M/C797S triple mutant (unknown origin) (696 to 1022 residues) expressed in insect cells assessed as de... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Mus musculus) | BDBM50505841

(CHEMBL4532034 | US11174245, # I-018)Show SMILES COc1ccncc1-c1cc(ccn1)C(=O)\N=c1/[nH]c2ccccc2n1CC(C)(C)O Show InChI InChI=1S/C23H23N5O3/c1-23(2,30)14-28-19-7-5-4-6-17(19)26-22(28)27-21(29)15-8-11-25-18(12-15)16-13-24-10-9-20(16)31-3/h4-13,30H,14H2,1-3H3,(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Mus musculus) | BDBM50505846

(CHEMBL4562138)Show SMILES C[C@@H]1CCCOc2c(cnn2C)-c2cc(cc(C)n2)C(=O)\N=C2/Nc3ccccc3N2C1 |r,c:23| Show InChI InChI=1S/C24H26N6O2/c1-15-7-6-10-32-23-18(13-25-29(23)3)20-12-17(11-16(2)26-20)22(31)28-24-27-19-8-4-5-9-21(19)30(24)14-15/h4-5,8-9,11-13,15H,6-7,10,14H2,1-3H3,(H,27,28,31)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Mus musculus) | BDBM50505842
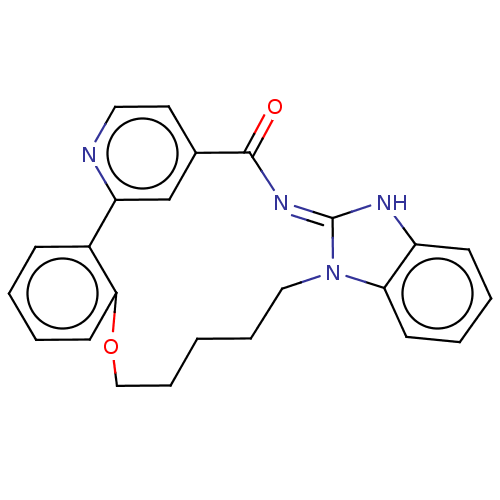
(CHEMBL4519157)Show SMILES O=C1\N=C2/Nc3ccccc3N2CCCCCOc2ccccc2-c2cc1ccn2 |c:2| Show InChI InChI=1S/C24H22N4O2/c29-23-17-12-13-25-20(16-17)18-8-2-5-11-22(18)30-15-7-1-6-14-28-21-10-4-3-9-19(21)26-24(28)27-23/h2-5,8-13,16H,1,6-7,14-15H2,(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50106343
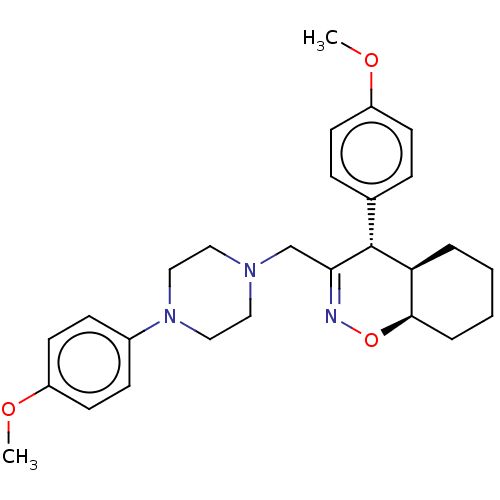
(CHEMBL3596280)Show SMILES [H][C@@]12CCCC[C@]1([H])[C@H](C(CN1CCN(CC1)c1ccc(OC)cc1)=NO2)c1ccc(OC)cc1 |r,c:27| Show InChI InChI=1S/C27H35N3O3/c1-31-22-11-7-20(8-12-22)27-24-5-3-4-6-26(24)33-28-25(27)19-29-15-17-30(18-16-29)21-9-13-23(32-2)14-10-21/h7-14,24,26-27H,3-6,15-19H2,1-2H3/t24-,26+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50106341
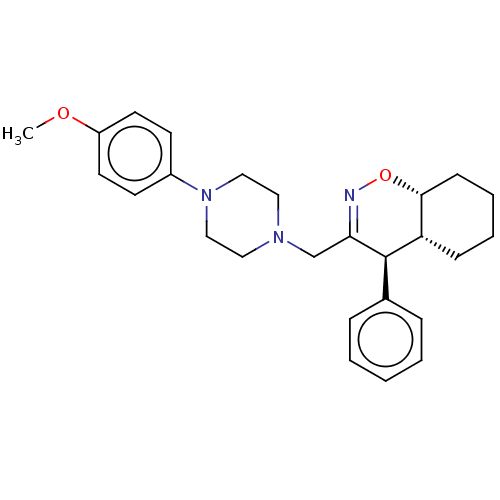
(CHEMBL3596282)Show SMILES [H][C@@]12CCCC[C@]1([H])[C@H](C(CN1CCN(CC1)c1ccc(OC)cc1)=NO2)c1ccccc1 |r,c:27| Show InChI InChI=1S/C26H33N3O2/c1-30-22-13-11-21(12-14-22)29-17-15-28(16-18-29)19-24-26(20-7-3-2-4-8-20)23-9-5-6-10-25(23)31-27-24/h2-4,7-8,11-14,23,25-26H,5-6,9-10,15-19H2,1H3/t23-,25+,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505837
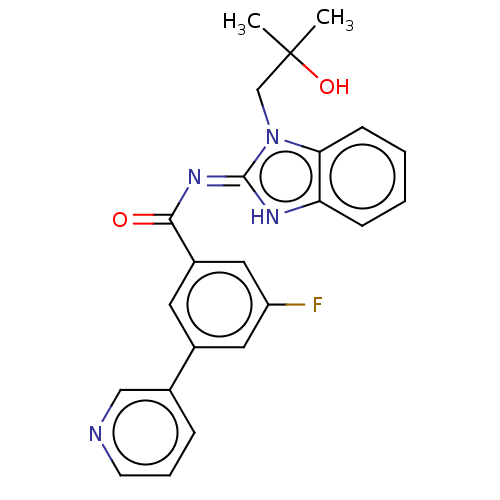
(CHEMBL4591327)Show SMILES CC(C)(O)Cn1c2ccccc2[nH]\c1=N/C(=O)c1cc(F)cc(c1)-c1cccnc1 Show InChI InChI=1S/C23H21FN4O2/c1-23(2,30)14-28-20-8-4-3-7-19(20)26-22(28)27-21(29)17-10-16(11-18(24)12-17)15-6-5-9-25-13-15/h3-13,30H,14H2,1-2H3,(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of GST-fusion tagged EGFR L858R/T790M/C797S triple mutant (unknown origin) (696 to 1022 residues) expressed in insect cells assessed as de... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Mus musculus) | BDBM50505840

(CHEMBL4550104)Show SMILES Cc1cc2cc(n1)-c1cnn(C)c1OCCCCCN1\C(Nc3ccccc13)=N\C2=O |t:31| Show InChI InChI=1S/C23H24N6O2/c1-15-12-16-13-19(25-15)17-14-24-28(2)22(17)31-11-7-3-6-10-29-20-9-5-4-8-18(20)26-23(29)27-21(16)30/h4-5,8-9,12-14H,3,6-7,10-11H2,1-2H3,(H,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50106342

(CHEMBL3596281)Show SMILES COc1ccc(cc1)N1CCN(CC2=NOC(C)(C)CC2c2ccccc2)CC1 |t:14| Show InChI InChI=1S/C24H31N3O2/c1-24(2)17-22(19-7-5-4-6-8-19)23(25-29-24)18-26-13-15-27(16-14-26)20-9-11-21(28-3)12-10-20/h4-12,22H,13-18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505844

(CHEMBL4444231)Show SMILES CC(C)(O)Cn1c2ccccc2[nH]\c1=N/C(=O)c1cccc(c1)-c1cccnc1 Show InChI InChI=1S/C23H22N4O2/c1-23(2,29)15-27-20-11-4-3-10-19(20)25-22(27)26-21(28)17-8-5-7-16(13-17)18-9-6-12-24-14-18/h3-14,29H,15H2,1-2H3,(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of GST-fusion tagged EGFR L858R/T790M/C797S triple mutant (unknown origin) (696 to 1022 residues) expressed in insect cells assessed as de... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Mus musculus) | BDBM50505843

(CHEMBL4449589 | US11174245, # I-235)Show SMILES CC(C)(O)Cn1c2ccccc2[nH]\c1=N/C(=O)c1ccnc(c1)-c1cccnc1 Show InChI InChI=1S/C22H21N5O2/c1-22(2,29)14-27-19-8-4-3-7-17(19)25-21(27)26-20(28)15-9-11-24-18(12-15)16-6-5-10-23-13-16/h3-13,29H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50106333
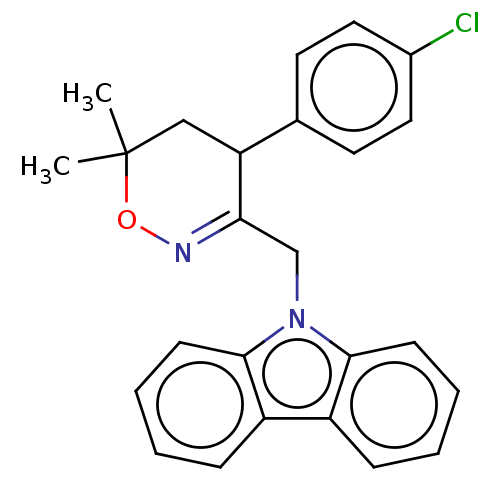
(CHEMBL3596289)Show SMILES CC1(C)CC(C(Cn2c3ccccc3c3ccccc23)=NO1)c1ccc(Cl)cc1 |c:22| Show InChI InChI=1S/C25H23ClN2O/c1-25(2)15-21(17-11-13-18(26)14-12-17)22(27-29-25)16-28-23-9-5-3-7-19(23)20-8-4-6-10-24(20)28/h3-14,21H,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50106338
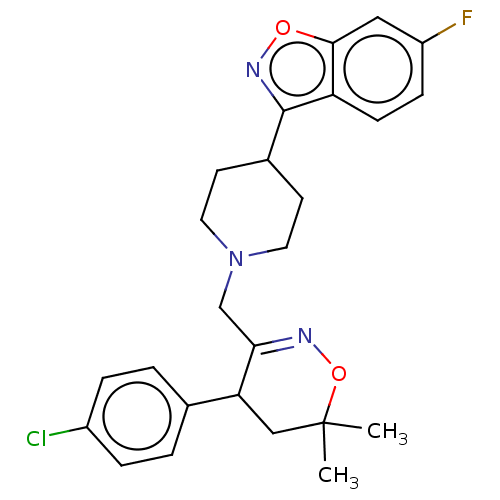
(CHEMBL3596285)Show SMILES CC1(C)CC(C(CN2CCC(CC2)c2noc3cc(F)ccc23)=NO1)c1ccc(Cl)cc1 |c:25| Show InChI InChI=1S/C25H27ClFN3O2/c1-25(2)14-21(16-3-5-18(26)6-4-16)22(28-32-25)15-30-11-9-17(10-12-30)24-20-8-7-19(27)13-23(20)31-29-24/h3-8,13,17,21H,9-12,14-15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50106339

(CHEMBL3596284)Show SMILES [H][C@@]12CCCC[C@]1([H])[C@H](C(CN1CCC(CC1)c1noc3cc(F)ccc13)=NO2)c1ccccc1 |r,c:30| Show InChI InChI=1S/C27H30FN3O2/c28-20-10-11-22-25(16-20)33-30-27(22)19-12-14-31(15-13-19)17-23-26(18-6-2-1-3-7-18)21-8-4-5-9-24(21)32-29-23/h1-3,6-7,10-11,16,19,21,24,26H,4-5,8-9,12-15,17H2/t21-,24+,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Mus musculus) | BDBM50505839

(CHEMBL4525935 | US11174245, # I-001)Show SMILES Cn1cc(cn1)-c1cc(ccn1)C(=O)\N=c1/[nH]c2ccccc2n1CC(C)(C)O Show InChI InChI=1S/C21H22N6O2/c1-21(2,29)13-27-18-7-5-4-6-16(18)24-20(27)25-19(28)14-8-9-22-17(10-14)15-11-23-26(3)12-15/h4-12,29H,13H2,1-3H3,(H,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50106335
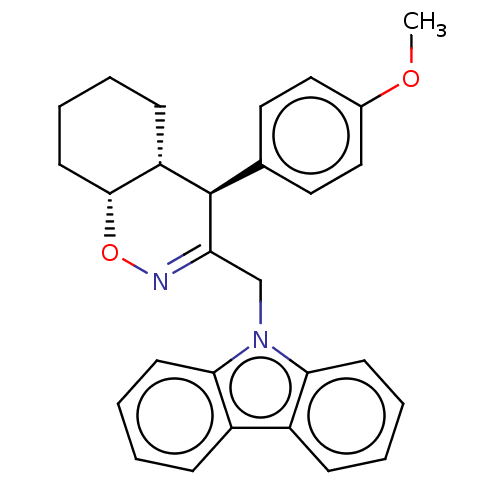
(CHEMBL3596288)Show SMILES [H][C@@]12CCCC[C@]1([H])[C@H](C(Cn1c3ccccc3c3ccccc13)=NO2)c1ccc(OC)cc1 |r,c:27| Show InChI InChI=1S/C28H28N2O2/c1-31-20-16-14-19(15-17-20)28-23-10-4-7-13-27(23)32-29-24(28)18-30-25-11-5-2-8-21(25)22-9-3-6-12-26(22)30/h2-3,5-6,8-9,11-12,14-17,23,27-28H,4,7,10,13,18H2,1H3/t23-,27+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184973
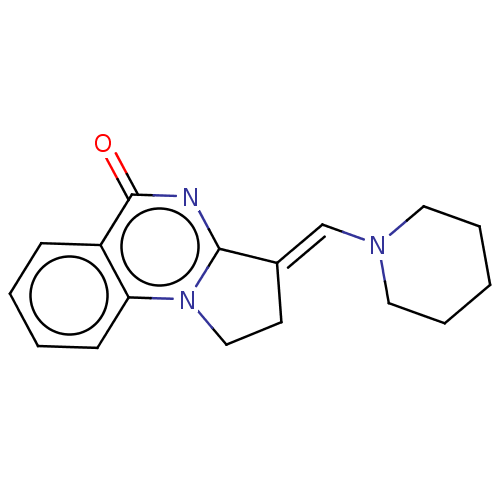
(CHEMBL3822828)Show InChI InChI=1S/C17H19N3O/c21-17-14-6-2-3-7-15(14)20-11-8-13(16(20)18-17)12-19-9-4-1-5-10-19/h2-3,6-7,12H,1,4-5,8-11H2/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50106337

(CHEMBL3596286)Show SMILES CC1(C)CC(C(CN2CCN(CC2)c2cccc(Cl)c2Cl)=NO1)c1ccccc1 |c:22| Show InChI InChI=1S/C23H27Cl2N3O/c1-23(2)15-18(17-7-4-3-5-8-17)20(26-29-23)16-27-11-13-28(14-12-27)21-10-6-9-19(24)22(21)25/h3-10,18H,11-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184975
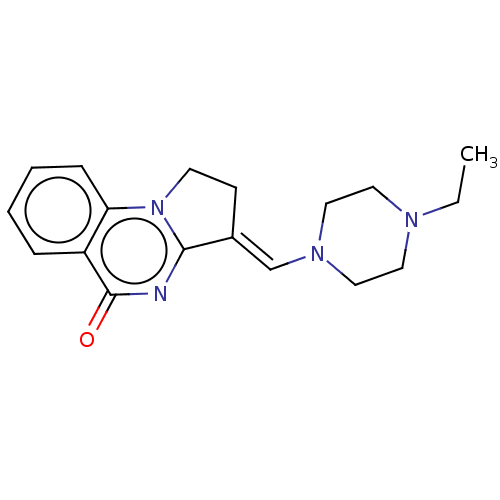
(CHEMBL3823214)Show InChI InChI=1S/C18H22N4O/c1-2-20-9-11-21(12-10-20)13-14-7-8-22-16-6-4-3-5-15(16)18(23)19-17(14)22/h3-6,13H,2,7-12H2,1H3/b14-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184974
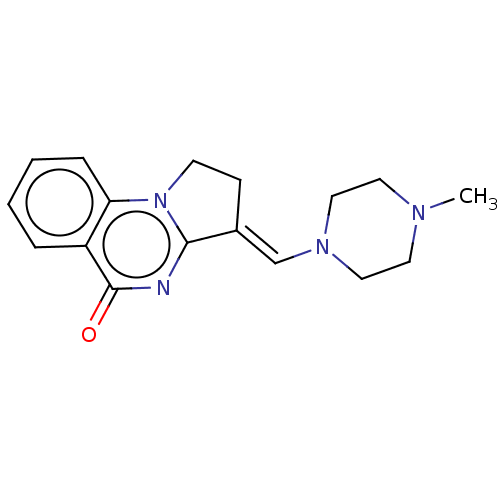
(CHEMBL3823057)Show InChI InChI=1S/C17H20N4O/c1-19-8-10-20(11-9-19)12-13-6-7-21-15-5-3-2-4-14(15)17(22)18-16(13)21/h2-5,12H,6-11H2,1H3/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184972
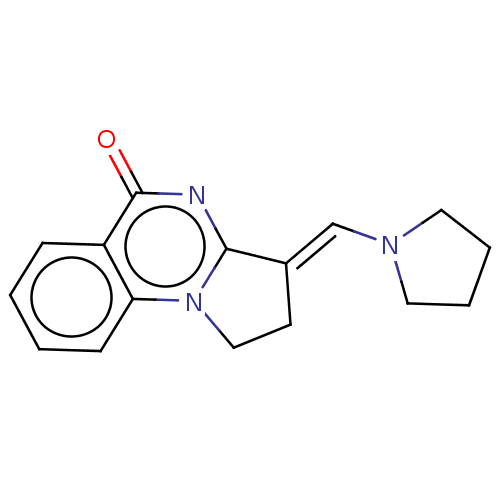
(CHEMBL3823525)Show InChI InChI=1S/C16H17N3O/c20-16-13-5-1-2-6-14(13)19-10-7-12(15(19)17-16)11-18-8-3-4-9-18/h1-2,5-6,11H,3-4,7-10H2/b12-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50106336
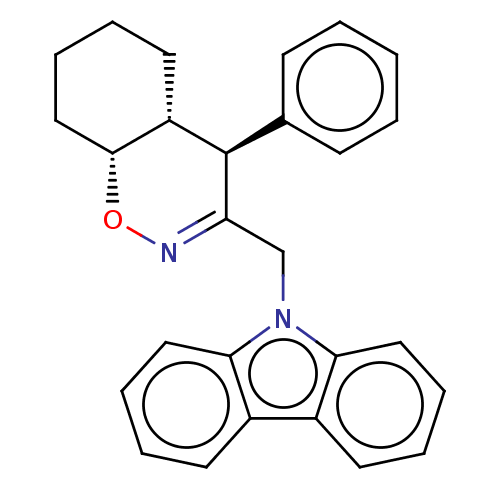
(CHEMBL3596287)Show SMILES [H][C@@]12CCCC[C@]1([H])[C@H](C(Cn1c3ccccc3c3ccccc13)=NO2)c1ccccc1 |r,c:27| Show InChI InChI=1S/C27H26N2O/c1-2-10-19(11-3-1)27-22-14-6-9-17-26(22)30-28-23(27)18-29-24-15-7-4-12-20(24)21-13-5-8-16-25(21)29/h1-5,7-8,10-13,15-16,22,26-27H,6,9,14,17-18H2/t22-,26+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505841

(CHEMBL4532034 | US11174245, # I-018)Show SMILES COc1ccncc1-c1cc(ccn1)C(=O)\N=c1/[nH]c2ccccc2n1CC(C)(C)O Show InChI InChI=1S/C23H23N5O3/c1-23(2,30)14-28-19-7-5-4-6-17(19)26-22(28)27-21(29)15-8-11-25-18(12-15)16-13-24-10-9-20(16)31-3/h4-13,30H,14H2,1-3H3,(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged wild type EGFR expressed in baculovirus expression system assessed as decrease in phosphorylation of ULigh... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Mus musculus) | BDBM50505836

(CHEMBL4434788 | US11174245, # I-064)Show SMILES CC(C)(O)Cn1c2ccccc2[nH]\c1=N/C(=O)c1ccnc(c1)-c1ccccc1 Show InChI InChI=1S/C23H22N4O2/c1-23(2,29)15-27-20-11-7-6-10-18(20)25-22(27)26-21(28)17-12-13-24-19(14-17)16-8-4-3-5-9-16/h3-14,29H,15H2,1-2H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR in mouse BAF3 cells assessed as reduction in cell proliferation incubated for 72 hrs by Celltiter-Glo luminescent cell v... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50106340
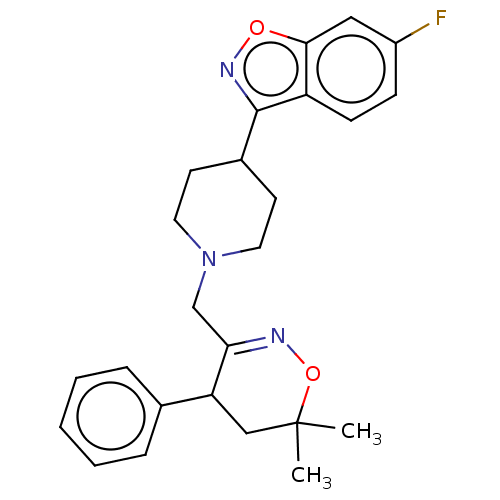
(CHEMBL3596283)Show SMILES CC1(C)CC(C(CN2CCC(CC2)c2noc3cc(F)ccc23)=NO1)c1ccccc1 |c:25| Show InChI InChI=1S/C25H28FN3O2/c1-25(2)15-21(17-6-4-3-5-7-17)22(27-31-25)16-29-12-10-18(11-13-29)24-20-9-8-19(26)14-23(20)30-28-24/h3-9,14,18,21H,10-13,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184971
(CHEMBL3823210)Show InChI InChI=1S/C14H15N3O/c1-16(2)9-10-7-8-17-12-6-4-3-5-11(12)14(18)15-13(10)17/h3-6,9H,7-8H2,1-2H3/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184976
(CHEMBL3823365)Show InChI InChI=1S/C18H22N4O/c1-20-8-4-9-21(12-11-20)13-14-7-10-22-16-6-3-2-5-15(16)18(23)19-17(14)22/h2-3,5-6,13H,4,7-12H2,1H3/b14-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50106341

(CHEMBL3596282)Show SMILES [H][C@@]12CCCC[C@]1([H])[C@H](C(CN1CCN(CC1)c1ccc(OC)cc1)=NO2)c1ccccc1 |r,c:27| Show InChI InChI=1S/C26H33N3O2/c1-30-22-13-11-21(12-14-22)29-17-15-28(16-18-29)19-24-26(20-7-3-2-4-8-20)23-9-5-6-10-25(23)31-27-24/h2-4,7-8,11-14,23,25-26H,5-6,9-10,15-19H2,1H3/t23-,25+,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50106340

(CHEMBL3596283)Show SMILES CC1(C)CC(C(CN2CCC(CC2)c2noc3cc(F)ccc23)=NO1)c1ccccc1 |c:25| Show InChI InChI=1S/C25H28FN3O2/c1-25(2)15-21(17-6-4-3-5-7-17)22(27-31-25)16-29-12-10-18(11-13-29)24-20-9-8-19(26)14-23(20)30-28-24/h3-9,14,18,21H,10-13,15-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50106338

(CHEMBL3596285)Show SMILES CC1(C)CC(C(CN2CCC(CC2)c2noc3cc(F)ccc23)=NO1)c1ccc(Cl)cc1 |c:25| Show InChI InChI=1S/C25H27ClFN3O2/c1-25(2)14-21(16-3-5-18(26)6-4-16)22(28-32-25)15-30-11-9-17(10-12-30)24-20-8-7-19(27)13-23(20)31-29-24/h3-8,13,17,21H,9-12,14-15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50106343

(CHEMBL3596280)Show SMILES [H][C@@]12CCCC[C@]1([H])[C@H](C(CN1CCN(CC1)c1ccc(OC)cc1)=NO2)c1ccc(OC)cc1 |r,c:27| Show InChI InChI=1S/C27H35N3O3/c1-31-22-11-7-20(8-12-22)27-24-5-3-4-6-26(24)33-28-25(27)19-29-15-17-30(18-16-29)21-9-13-23(32-2)14-10-21/h7-14,24,26-27H,3-6,15-19H2,1-2H3/t24-,26+,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50106333

(CHEMBL3596289)Show SMILES CC1(C)CC(C(Cn2c3ccccc3c3ccccc23)=NO1)c1ccc(Cl)cc1 |c:22| Show InChI InChI=1S/C25H23ClN2O/c1-25(2)15-21(17-11-13-18(26)14-12-17)22(27-29-25)16-28-23-9-5-3-7-19(23)20-8-4-6-10-24(20)28/h3-14,21H,15-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50106339

(CHEMBL3596284)Show SMILES [H][C@@]12CCCC[C@]1([H])[C@H](C(CN1CCC(CC1)c1noc3cc(F)ccc13)=NO2)c1ccccc1 |r,c:30| Show InChI InChI=1S/C27H30FN3O2/c28-20-10-11-22-25(16-20)33-30-27(22)19-12-14-31(15-13-19)17-23-26(18-6-2-1-3-7-18)21-8-4-5-9-24(21)32-29-23/h1-3,6-7,10-11,16,19,21,24,26H,4-5,8-9,12-15,17H2/t21-,24+,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50106342

(CHEMBL3596281)Show SMILES COc1ccc(cc1)N1CCN(CC2=NOC(C)(C)CC2c2ccccc2)CC1 |t:14| Show InChI InChI=1S/C24H31N3O2/c1-24(2)17-22(19-7-5-4-6-8-19)23(25-29-24)18-26-13-15-27(16-14-26)20-9-11-21(28-3)12-10-20/h4-12,22H,13-18H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50106335

(CHEMBL3596288)Show SMILES [H][C@@]12CCCC[C@]1([H])[C@H](C(Cn1c3ccccc3c3ccccc13)=NO2)c1ccc(OC)cc1 |r,c:27| Show InChI InChI=1S/C28H28N2O2/c1-31-20-16-14-19(15-17-20)28-23-10-4-7-13-27(23)32-29-24(28)18-30-25-11-5-2-8-21(25)22-9-3-6-12-26(22)30/h2-3,5-6,8-9,11-12,14-17,23,27-28H,4,7,10,13,18H2,1H3/t23-,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50106337

(CHEMBL3596286)Show SMILES CC1(C)CC(C(CN2CCN(CC2)c2cccc(Cl)c2Cl)=NO1)c1ccccc1 |c:22| Show InChI InChI=1S/C23H27Cl2N3O/c1-23(2)15-18(17-7-4-3-5-8-17)20(26-29-23)16-27-11-13-28(14-12-27)21-10-6-9-19(24)22(21)25/h3-10,18H,11-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50106336

(CHEMBL3596287)Show SMILES [H][C@@]12CCCC[C@]1([H])[C@H](C(Cn1c3ccccc3c3ccccc13)=NO2)c1ccccc1 |r,c:27| Show InChI InChI=1S/C27H26N2O/c1-2-10-19(11-3-1)27-22-14-6-9-17-26(22)30-28-23(27)18-29-24-15-7-4-12-20(24)21-13-5-8-16-25(21)29/h1-5,7-8,10-13,15-16,22,26-27H,6,9,14,17-18H2/t22-,26+,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bangalore University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Bioorg Med Chem Lett 25: 2931-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.047
BindingDB Entry DOI: 10.7270/Q2D220D5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505843

(CHEMBL4449589 | US11174245, # I-235)Show SMILES CC(C)(O)Cn1c2ccccc2[nH]\c1=N/C(=O)c1ccnc(c1)-c1cccnc1 Show InChI InChI=1S/C22H21N5O2/c1-22(2,29)14-27-19-8-4-3-7-17(19)25-21(27)26-20(28)15-9-11-24-18(12-15)16-6-5-10-23-13-16/h3-13,29H,14H2,1-2H3,(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged wild type EGFR expressed in baculovirus expression system assessed as decrease in phosphorylation of ULigh... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505844

(CHEMBL4444231)Show SMILES CC(C)(O)Cn1c2ccccc2[nH]\c1=N/C(=O)c1cccc(c1)-c1cccnc1 Show InChI InChI=1S/C23H22N4O2/c1-23(2,29)15-27-20-11-4-3-10-19(20)25-22(27)26-21(28)17-8-5-7-16(13-17)18-9-6-12-24-14-18/h3-14,29H,15H2,1-2H3,(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged wild type EGFR expressed in baculovirus expression system assessed as decrease in phosphorylation of ULigh... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505836

(CHEMBL4434788 | US11174245, # I-064)Show SMILES CC(C)(O)Cn1c2ccccc2[nH]\c1=N/C(=O)c1ccnc(c1)-c1ccccc1 Show InChI InChI=1S/C23H22N4O2/c1-23(2,29)15-27-20-11-7-6-10-18(20)25-22(27)26-21(28)17-12-13-24-19(14-17)16-8-4-3-5-9-16/h3-14,29H,15H2,1-2H3,(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged wild type EGFR expressed in baculovirus expression system assessed as decrease in phosphorylation of ULigh... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505839

(CHEMBL4525935 | US11174245, # I-001)Show SMILES Cn1cc(cn1)-c1cc(ccn1)C(=O)\N=c1/[nH]c2ccccc2n1CC(C)(C)O Show InChI InChI=1S/C21H22N6O2/c1-21(2,29)13-27-18-7-5-4-6-16(18)24-20(27)25-19(28)14-8-9-22-17(10-14)15-11-23-26(3)12-15/h4-12,29H,13H2,1-3H3,(H,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged wild type EGFR expressed in baculovirus expression system assessed as decrease in phosphorylation of ULigh... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505837

(CHEMBL4591327)Show SMILES CC(C)(O)Cn1c2ccccc2[nH]\c1=N/C(=O)c1cc(F)cc(c1)-c1cccnc1 Show InChI InChI=1S/C23H21FN4O2/c1-23(2,30)14-28-20-8-4-3-7-19(20)26-22(28)27-21(29)17-10-16(11-18(24)12-17)15-6-5-9-25-13-15/h3-13,30H,14H2,1-2H3,(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH & Co KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged wild type EGFR expressed in baculovirus expression system assessed as decrease in phosphorylation of ULigh... |
J Med Chem 62: 10272-10293 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01169
BindingDB Entry DOI: 10.7270/Q2XD14ZQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data