 Found 411 hits with Last Name = 'fukuda' and Initial = 'h'
Found 411 hits with Last Name = 'fukuda' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50506970
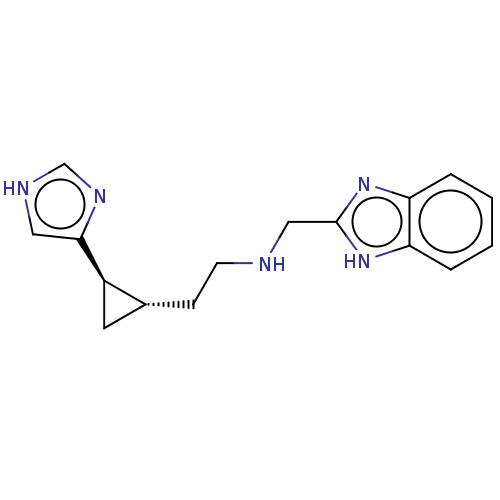
(CHEMBL4592695)Show SMILES C(C[C@@H]1C[C@H]1c1c[nH]cn1)NCc1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C16H19N5/c1-2-4-14-13(3-1)20-16(21-14)9-17-6-5-11-7-12(11)15-8-18-10-19-15/h1-4,8,10-12,17H,5-7,9H2,(H,18,19)(H,20,21)/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cell membranes after 30 mins by liquid scint... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50506970

(CHEMBL4592695)Show SMILES C(C[C@@H]1C[C@H]1c1c[nH]cn1)NCc1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C16H19N5/c1-2-4-14-13(3-1)20-16(21-14)9-17-6-5-11-7-12(11)15-8-18-10-19-15/h1-4,8,10-12,17H,5-7,9H2,(H,18,19)(H,20,21)/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cell membranes after 30 mins by liquid scintillation anal... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50506967

(CHEMBL4529871)Show SMILES C([C@@H]1C[C@H]1c1c[nH]cn1)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C14H14N4/c1-2-4-12-11(3-1)17-14(18-12)6-9-5-10(9)13-7-15-8-16-13/h1-4,7-10H,5-6H2,(H,15,16)(H,17,18)/t9-,10+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cell membranes after 30 mins by liquid scint... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50506967

(CHEMBL4529871)Show SMILES C([C@@H]1C[C@H]1c1c[nH]cn1)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C14H14N4/c1-2-4-12-11(3-1)17-14(18-12)6-9-5-10(9)13-7-15-8-16-13/h1-4,7-10H,5-6H2,(H,15,16)(H,17,18)/t9-,10+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cell membranes after 30 mins by liquid scintillation anal... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50506973

(CHEMBL4545523)Show InChI InChI=1S/C15H22ClN3/c16-14-3-1-12(2-4-14)10-18-11-13-9-15(13)19-7-5-17-6-8-19/h1-4,13,15,17-18H,5-11H2/t13-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cell membranes after 30 mins by liquid scint... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50506974

(CHEMBL4436775)Show SMILES Clc1ccc(CNCC[C@@H]2C[C@H]2c2nc3cc(Cl)ccc3[nH]2)cc1 |r| Show InChI InChI=1S/C19H19Cl2N3/c20-14-3-1-12(2-4-14)11-22-8-7-13-9-16(13)19-23-17-6-5-15(21)10-18(17)24-19/h1-6,10,13,16,22H,7-9,11H2,(H,23,24)/t13-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cell membranes after 30 mins by liquid scint... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50506969

(CHEMBL4472944)Show SMILES Clc1ccc(CNC[C@@H]2C[C@@H]2c2cc3cc(Cl)ccc3[nH]2)cc1 |r| Show InChI InChI=1S/C19H18Cl2N2/c20-15-3-1-12(2-4-15)10-22-11-14-8-17(14)19-9-13-7-16(21)5-6-18(13)23-19/h1-7,9,14,17,22-23H,8,10-11H2/t14-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cell membranes after 30 mins by liquid scint... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50506975
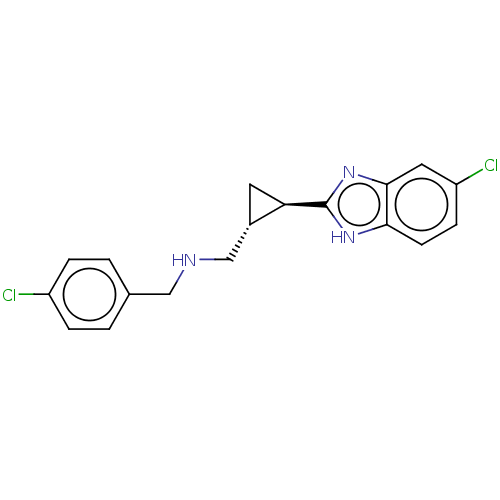
(CHEMBL4465258)Show SMILES Clc1ccc(CNC[C@@H]2C[C@H]2c2nc3cc(Cl)ccc3[nH]2)cc1 |r| Show InChI InChI=1S/C18H17Cl2N3/c19-13-3-1-11(2-4-13)9-21-10-12-7-15(12)18-22-16-6-5-14(20)8-17(16)23-18/h1-6,8,12,15,21H,7,9-10H2,(H,22,23)/t12-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cell membranes after 30 mins by liquid scintillation anal... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50506972

(CHEMBL4475673)Show InChI InChI=1S/C16H24ClN3/c17-15-3-1-13(2-4-15)12-19-6-5-14-11-16(14)20-9-7-18-8-10-20/h1-4,14,16,18-19H,5-12H2/t14-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cell membranes after 30 mins by liquid scintillation anal... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50506976
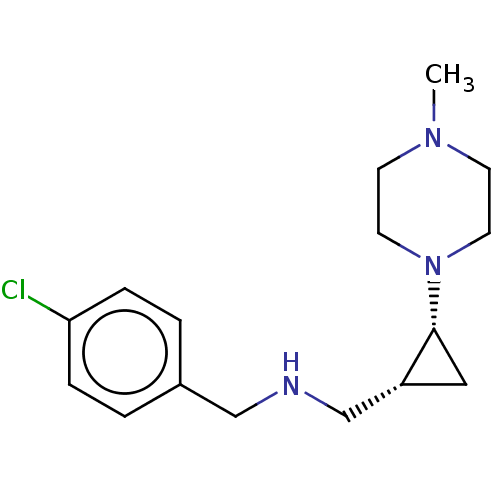
(CHEMBL4469188)Show SMILES CN1CCN(CC1)[C@@H]1C[C@@H]1CNCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C16H24ClN3/c1-19-6-8-20(9-7-19)16-10-14(16)12-18-11-13-2-4-15(17)5-3-13/h2-5,14,16,18H,6-12H2,1H3/t14-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cell membranes after 30 mins by liquid scintillation anal... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50506975

(CHEMBL4465258)Show SMILES Clc1ccc(CNC[C@@H]2C[C@H]2c2nc3cc(Cl)ccc3[nH]2)cc1 |r| Show InChI InChI=1S/C18H17Cl2N3/c19-13-3-1-11(2-4-13)9-21-10-12-7-15(12)18-22-16-6-5-14(20)8-17(16)23-18/h1-6,8,12,15,21H,7,9-10H2,(H,22,23)/t12-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cell membranes after 30 mins by liquid scint... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50506973

(CHEMBL4545523)Show InChI InChI=1S/C15H22ClN3/c16-14-3-1-12(2-4-14)10-18-11-13-9-15(13)19-7-5-17-6-8-19/h1-4,13,15,17-18H,5-11H2/t13-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cell membranes after 30 mins by liquid scintillation anal... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50506976

(CHEMBL4469188)Show SMILES CN1CCN(CC1)[C@@H]1C[C@@H]1CNCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C16H24ClN3/c1-19-6-8-20(9-7-19)16-10-14(16)12-18-11-13-2-4-15(17)5-3-13/h2-5,14,16,18H,6-12H2,1H3/t14-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cell membranes after 30 mins by liquid scint... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50506968
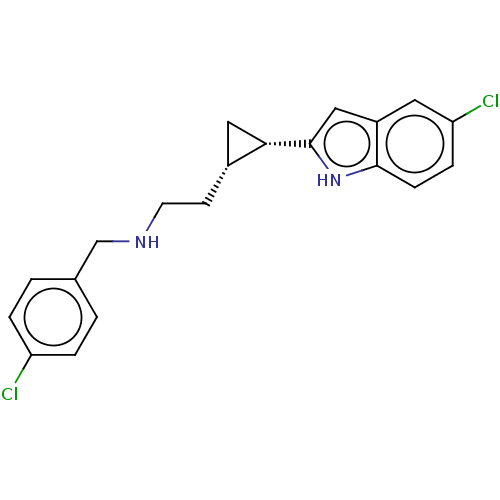
(CHEMBL4585991)Show SMILES Clc1ccc(CNCC[C@@H]2C[C@@H]2c2cc3cc(Cl)ccc3[nH]2)cc1 |r| Show InChI InChI=1S/C20H20Cl2N2/c21-16-3-1-13(2-4-16)12-23-8-7-14-10-18(14)20-11-15-9-17(22)5-6-19(15)24-20/h1-6,9,11,14,18,23-24H,7-8,10,12H2/t14-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cell membranes after 30 mins by liquid scint... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50506974

(CHEMBL4436775)Show SMILES Clc1ccc(CNCC[C@@H]2C[C@H]2c2nc3cc(Cl)ccc3[nH]2)cc1 |r| Show InChI InChI=1S/C19H19Cl2N3/c20-14-3-1-12(2-4-14)11-22-8-7-13-9-16(13)19-23-17-6-5-15(21)10-18(17)24-19/h1-6,10,13,16,22H,7-9,11H2,(H,23,24)/t13-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cell membranes after 30 mins by liquid scintillation anal... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50506972

(CHEMBL4475673)Show InChI InChI=1S/C16H24ClN3/c17-15-3-1-13(2-4-15)12-19-6-5-14-11-16(14)20-9-7-18-8-10-20/h1-4,14,16,18-19H,5-12H2/t14-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cell membranes after 30 mins by liquid scint... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50506971

(CHEMBL4475476)Show SMILES CN1CCN(CC1)[C@@H]1C[C@@H]1CCNCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H26ClN3/c1-20-8-10-21(11-9-20)17-12-15(17)6-7-19-13-14-2-4-16(18)5-3-14/h2-5,15,17,19H,6-13H2,1H3/t15-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cell membranes after 30 mins by liquid scint... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50506969

(CHEMBL4472944)Show SMILES Clc1ccc(CNC[C@@H]2C[C@@H]2c2cc3cc(Cl)ccc3[nH]2)cc1 |r| Show InChI InChI=1S/C19H18Cl2N2/c20-15-3-1-12(2-4-15)10-22-11-14-8-17(14)19-9-13-7-16(21)5-6-18(13)23-19/h1-7,9,14,17,22-23H,8,10-11H2/t14-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cell membranes after 30 mins by liquid scintillation anal... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50506971

(CHEMBL4475476)Show SMILES CN1CCN(CC1)[C@@H]1C[C@@H]1CCNCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H26ClN3/c1-20-8-10-21(11-9-20)17-12-15(17)6-7-19-13-14-2-4-16(18)5-3-14/h2-5,15,17,19H,6-13H2,1H3/t15-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cell membranes after 30 mins by liquid scintillation anal... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50506968

(CHEMBL4585991)Show SMILES Clc1ccc(CNCC[C@@H]2C[C@@H]2c2cc3cc(Cl)ccc3[nH]2)cc1 |r| Show InChI InChI=1S/C20H20Cl2N2/c21-16-3-1-13(2-4-16)12-23-8-7-14-10-18(14)20-11-15-9-17(22)5-6-19(15)24-20/h1-6,9,11,14,18,23-24H,7-8,10,12H2/t14-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cell membranes after 30 mins by liquid scintillation anal... |
Bioorg Med Chem Lett 28: 3630-3633 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.041
BindingDB Entry DOI: 10.7270/Q2GX4FVC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082445
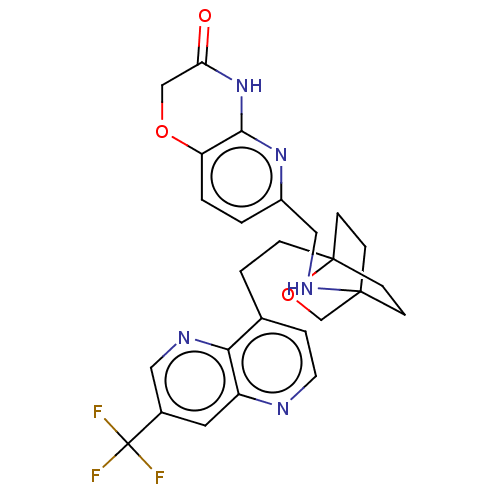
(CHEMBL3422978)Show SMILES FC(F)(F)c1cnc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2c1 |(-5.09,-.93,;-4.02,-1.54,;-4.02,-2.78,;-5.09,-2.16,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)17-11-19-22(31-12-17)16(4-10-30-19)3-5-25-8-6-24(7-9-25,15-37-25)32-13-18-1-2-20-23(33-18)34-21(35)14-36-20/h1-2,4,10-12,32H,3,5-9,13-15H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272596
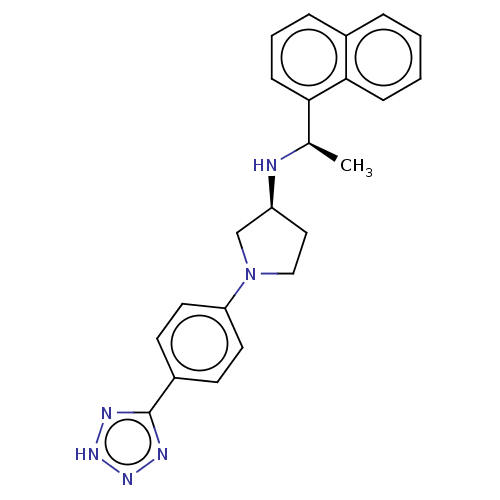
(CHEMBL4126450)Show SMILES Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(cc1)-c1nn[nH]n1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N6.ClH/c1-16(21-8-4-6-17-5-2-3-7-22(17)21)24-19-13-14-29(15-19)20-11-9-18(10-12-20)23-25-27-28-26-23;/h2-12,16,19,24H,13-15H2,1H3,(H,25,26,27,28);1H/t16-,19+;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082380
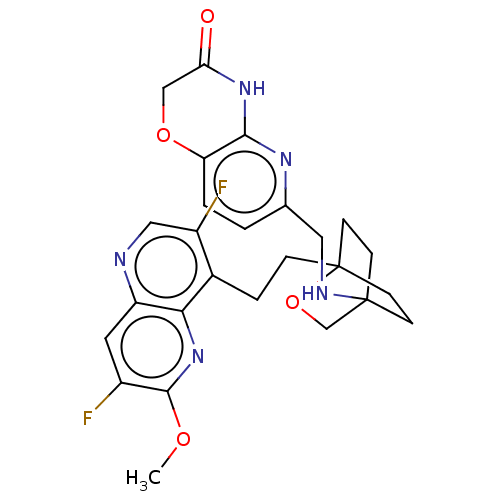
(CHEMBL3422952)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H27F2N5O4/c1-35-24-17(27)10-19-22(33-24)16(18(28)12-29-19)4-5-26-8-6-25(7-9-26,14-37-26)30-11-15-2-3-20-23(31-15)32-21(34)13-36-20/h2-3,10,12,30H,4-9,11,13-14H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082429

(CHEMBL3422970)Show SMILES Cc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-3.75,1.39,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O3/c1-17-19(13-28)12-21-24(31-17)18(5-11-29-21)4-6-27-9-7-26(8-10-27,16-36-27)30-14-20-2-3-22-25(32-20)33-23(34)15-35-22/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082385
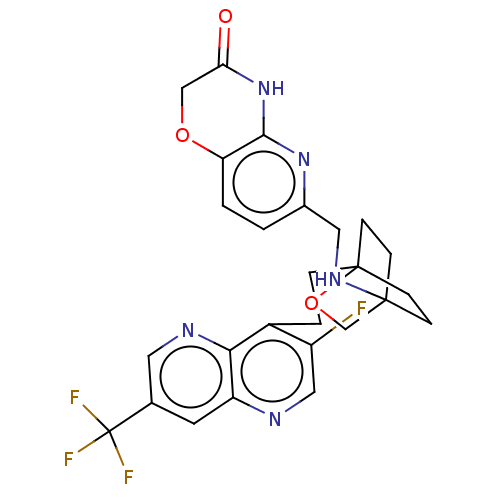
(CHEMBL3422959)Show SMILES Fc1cnc2cc(cnc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1)C(F)(F)F Show InChI InChI=1S/C26H25F4N5O3/c27-18-12-31-19-9-15(26(28,29)30)10-32-22(19)17(18)3-4-25-7-5-24(6-8-25,14-38-25)33-11-16-1-2-20-23(34-16)35-21(36)13-37-20/h1-2,9-10,12,33H,3-8,11,13-14H2,(H,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272602
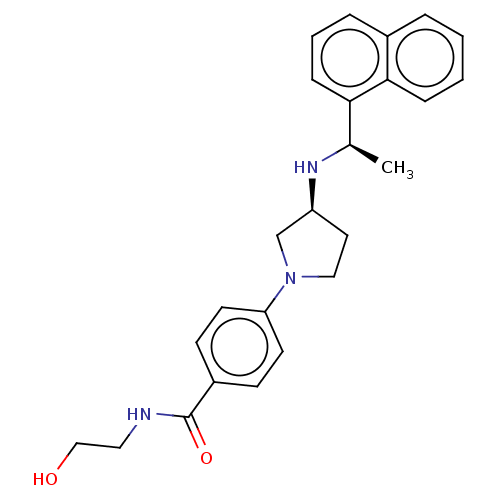
(CHEMBL4126877)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(cc1)C(=O)NCCO)c1cccc2ccccc12 |r| Show InChI InChI=1S/C25H29N3O2.2ClH/c1-18(23-8-4-6-19-5-2-3-7-24(19)23)27-21-13-15-28(17-21)22-11-9-20(10-12-22)25(30)26-14-16-29;;/h2-12,18,21,27,29H,13-17H2,1H3,(H,26,30);2*1H/t18-,21+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082440

(CHEMBL3422977)Show SMILES FC(F)(F)c1ccc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-5.09,.93,;-4.02,1.54,;-4.02,2.78,;-5.09,2.16,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)20-4-2-18-22(33-20)16(6-12-30-18)5-7-25-10-8-24(9-11-25,15-37-25)31-13-17-1-3-19-23(32-17)34-21(35)14-36-19/h1-4,6,12,31H,5,7-11,13-15H2,(H,32,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082382
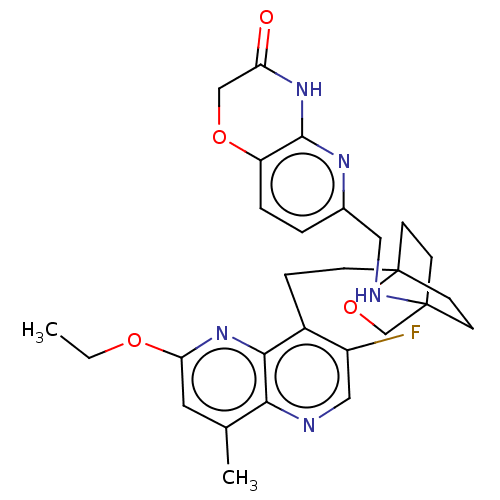
(CHEMBL3422954)Show SMILES CCOc1cc(C)c2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-5.09,3.71,;-4.02,3.09,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-2.77,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H32FN5O4/c1-3-36-23-12-17(2)24-25(34-23)19(20(29)14-30-24)6-7-28-10-8-27(9-11-28,16-38-28)31-13-18-4-5-21-26(32-18)33-22(35)15-37-21/h4-5,12,14,31H,3,6-11,13,15-16H2,1-2H3,(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082435
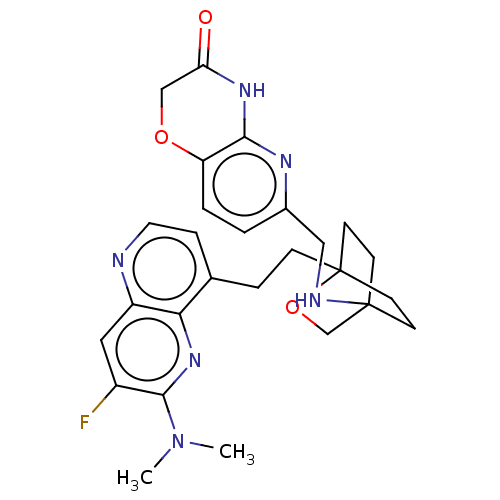
(CHEMBL3422976)Show SMILES CN(C)c1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-5.09,.93,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C27H31FN6O3/c1-34(2)25-19(28)13-20-23(33-25)17(6-12-29-20)5-7-27-10-8-26(9-11-27,16-37-27)30-14-18-3-4-21-24(31-18)32-22(35)15-36-21/h3-4,6,12-13,30H,5,7-11,14-16H2,1-2H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272605

(CHEMBL4128542)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCCN(C1)c1cccc(OC(F)(F)F)c1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H25F3N2O.2ClH/c1-17(22-13-4-8-18-7-2-3-12-23(18)22)28-19-9-6-14-29(16-19)20-10-5-11-21(15-20)30-24(25,26)27;;/h2-5,7-8,10-13,15,17,19,28H,6,9,14,16H2,1H3;2*1H/t17-,19-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082390
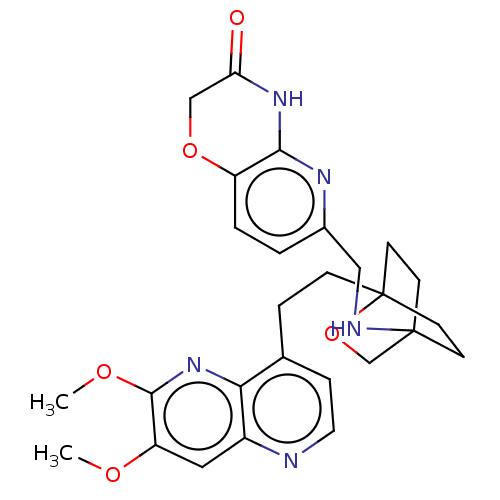
(CHEMBL3422964)Show SMILES COc1cc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2nc1OC |(-4.02,-2.78,;-4.02,-1.54,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,)| Show InChI InChI=1S/C27H31N5O5/c1-34-21-13-19-23(32-25(21)35-2)17(6-12-28-19)5-7-27-10-8-26(9-11-27,16-37-27)29-14-18-3-4-20-24(30-18)31-22(33)15-36-20/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082394

(CHEMBL3422966)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O4/c1-35-25-18(13-28)12-20-23(33-25)17(5-11-29-20)4-6-27-9-7-26(8-10-27,16-37-27)30-14-19-2-3-21-24(31-19)32-22(34)15-36-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082388
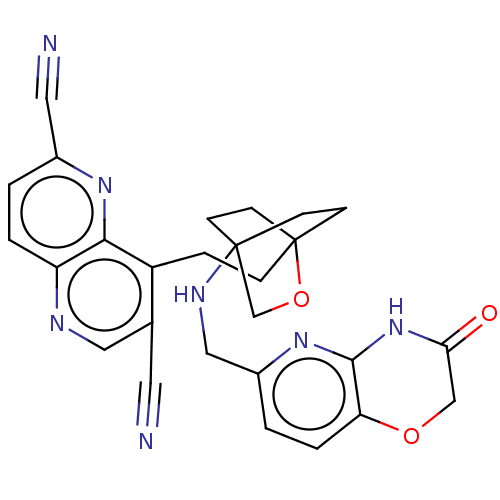
(CHEMBL3422962)Show SMILES O=C1COc2ccc(CNC34CCC(CCc5c(cnc6ccc(nc56)C#N)C#N)(CC3)OC4)nc2N1 |(10.96,15.37,;9.76,15.09,;8.71,16.22,;7.21,15.87,;6.76,14.39,;5.26,14.04,;4.8,12.56,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;-4.02,1.54,;-5.09,2.16,;4,1.54,;5.07,2.16,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,;7.37,11.8,;7.81,13.27,;9.31,13.62,)| Show InChI InChI=1S/C27H25N7O3/c28-11-17-13-30-21-3-1-18(12-29)32-24(21)20(17)5-6-27-9-7-26(8-10-27,16-37-27)31-14-19-2-4-22-25(33-19)34-23(35)15-36-22/h1-4,13,31H,5-10,14-16H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082383

(CHEMBL3422957)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1C(F)(F)F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-.93,;-4.02,-2.78,;-5.09,-2.16,)| Show InChI InChI=1S/C27H27F4N5O4/c1-38-24-17(27(29,30)31)10-19-22(36-24)16(18(28)12-32-19)4-5-26-8-6-25(7-9-26,14-40-26)33-11-15-2-3-20-23(34-15)35-21(37)13-39-20/h2-3,10,12,33H,4-9,11,13-14H2,1H3,(H,34,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082427
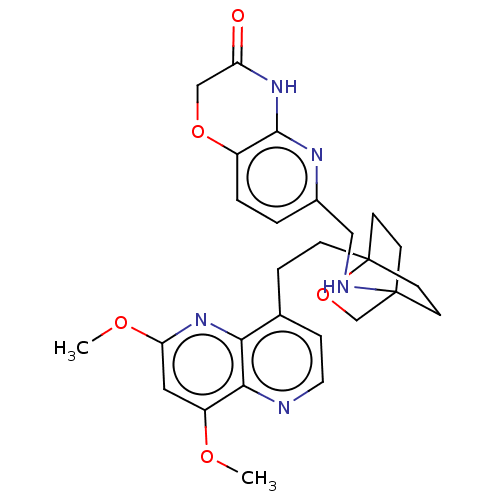
(CHEMBL3422968)Show SMILES COc1cc(OC)c2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-3.08,;-2.39,-3.71,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H31N5O5/c1-34-20-13-22(35-2)32-23-17(6-12-28-24(20)23)5-7-27-10-8-26(9-11-27,16-37-27)29-14-18-3-4-19-25(30-18)31-21(33)15-36-19/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082430
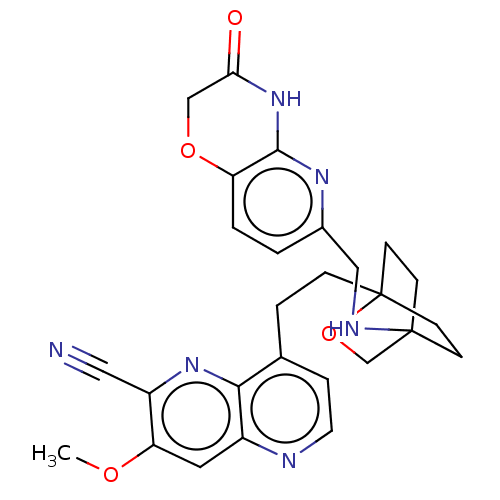
(CHEMBL3422971)Show SMILES COc1cc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2nc1C#N |(-4.02,-2.78,;-4.02,-1.54,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,;-2.69,.77,;-4.02,1.54,;-5.09,2.16,)| Show InChI InChI=1S/C27H28N6O4/c1-35-22-12-19-24(32-20(22)13-28)17(5-11-29-19)4-6-27-9-7-26(8-10-27,16-37-27)30-14-18-2-3-21-25(31-18)33-23(34)15-36-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272606

(CHEMBL4125917)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCCN(C1)c1cccc(c1)C(F)(F)F)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H25F3N2.2ClH/c1-17(22-13-4-8-18-7-2-3-12-23(18)22)28-20-10-6-14-29(16-20)21-11-5-9-19(15-21)24(25,26)27;;/h2-5,7-9,11-13,15,17,20,28H,6,10,14,16H2,1H3;2*1H/t17-,20-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082520

(CHEMBL3422983)Show SMILES COc1ccc2ncc(C#N)c(CC(O)C34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;4,1.54,;5.07,2.16,;1.33,1.54,;1.34,3.08,;2.67,3.85,;3.74,3.23,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H28N6O5/c1-36-23-5-3-19-24(33-23)18(16(11-28)12-29-19)10-21(34)27-8-6-26(7-9-27,15-38-27)30-13-17-2-4-20-25(31-17)32-22(35)14-37-20/h2-5,12,21,30,34H,6-10,13-15H2,1H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082432
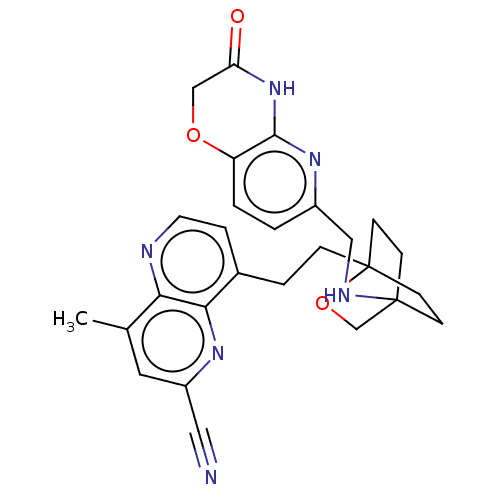
(CHEMBL3422973)Show SMILES Cc1cc(nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc12)C#N |(-1.33,-2.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-4.02,1.54,;-5.09,2.16,)| Show InChI InChI=1S/C27H28N6O3/c1-17-12-20(13-28)31-24-18(5-11-29-23(17)24)4-6-27-9-7-26(8-10-27,16-36-27)30-14-19-2-3-21-25(32-19)33-22(34)15-35-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082520

(CHEMBL3422983)Show SMILES COc1ccc2ncc(C#N)c(CC(O)C34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;4,1.54,;5.07,2.16,;1.33,1.54,;1.34,3.08,;2.67,3.85,;3.74,3.23,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H28N6O5/c1-36-23-5-3-19-24(33-23)18(16(11-28)12-29-19)10-21(34)27-8-6-26(7-9-27,15-38-27)30-13-17-2-4-20-25(31-17)32-22(35)14-37-20/h2-5,12,21,30,34H,6-10,13-15H2,1H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082434

(CHEMBL3422975)Show SMILES CN(C)c1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C(F)(F)F |(-4.02,2.78,;-4.02,1.54,;-5.09,.93,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-.93,;-4.02,-2.78,;-5.09,-2.16,)| Show InChI InChI=1S/C28H31F3N6O3/c1-37(2)25-19(28(29,30)31)13-20-23(36-25)17(6-12-32-20)5-7-27-10-8-26(9-11-27,16-40-27)33-14-18-3-4-21-24(34-18)35-22(38)15-39-21/h3-4,6,12-13,33H,5,7-11,14-16H2,1-2H3,(H,34,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082391
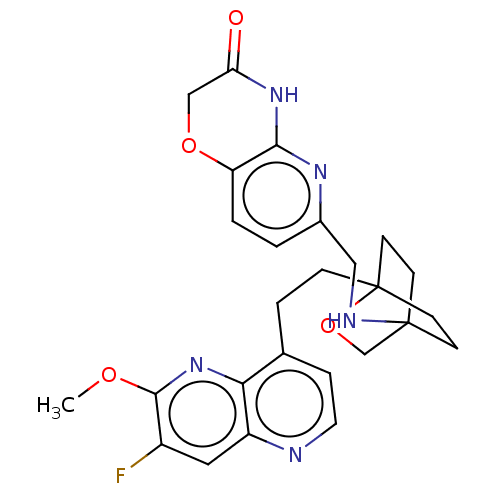
(CHEMBL3422965)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H28FN5O4/c1-34-24-18(27)12-19-22(32-24)16(5-11-28-19)4-6-26-9-7-25(8-10-26,15-36-26)29-13-17-2-3-20-23(30-17)31-21(33)14-35-20/h2-3,5,11-12,29H,4,6-10,13-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082381

(CHEMBL3422953)Show SMILES COc1cc(C)c2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-2.77,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-11-22(35-2)33-24-18(19(28)13-29-23(16)24)5-6-27-9-7-26(8-10-27,15-37-27)30-12-17-3-4-20-25(31-17)32-21(34)14-36-20/h3-4,11,13,30H,5-10,12,14-15H2,1-2H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082376

(CHEMBL3422948)Show SMILES Nc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-3.75,1.39,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H27FN6O3/c26-17-12-28-18-2-4-20(27)31-22(18)16(17)5-6-25-9-7-24(8-10-25,14-35-25)29-11-15-1-3-19-23(30-15)32-21(33)13-34-19/h1-4,12,29H,5-11,13-14H2,(H2,27,31)(H,30,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082387

(CHEMBL3422961)Show SMILES Fc1cnc2cc(Cl)cnc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1 |(3.74,1.39,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,)| Show InChI InChI=1S/C25H25ClFN5O3/c26-15-9-19-22(29-10-15)17(18(27)12-28-19)3-4-25-7-5-24(6-8-25,14-35-25)30-11-16-1-2-20-23(31-16)32-21(33)13-34-20/h1-2,9-10,12,30H,3-8,11,13-14H2,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082340
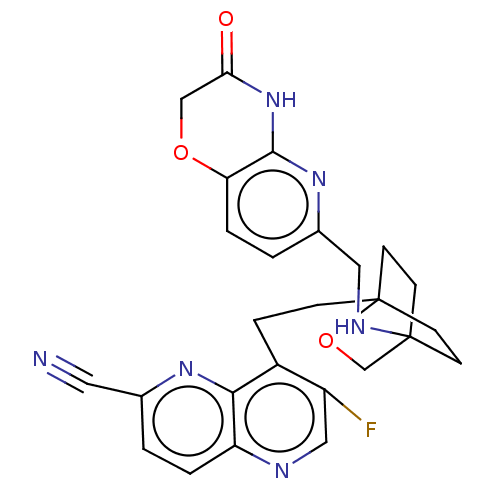
(CHEMBL3422947)Show SMILES Fc1cnc2ccc(nc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1)C#N |(-12.24,-4.65,;-12.56,-5.84,;-14.04,-6.24,;-14.44,-7.72,;-13.36,-8.81,;-13.75,-10.3,;-12.66,-11.41,;-11.17,-11.01,;-10.78,-9.5,;-11.87,-8.41,;-11.47,-6.93,;-9.98,-6.52,;-9.58,-5.03,;-8.11,-4.64,;-7.75,-3.13,;-6.28,-2.7,;-5.35,-3.86,;-5.52,-5.26,;-6.99,-5.7,;-6.24,-4.82,;-7.05,-3.51,;-4.02,-3.09,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-10.08,-12.1,;-9.21,-12.97,)| Show InChI InChI=1S/C26H25FN6O3/c27-19-13-29-20-3-1-16(11-28)31-23(20)18(19)5-6-26-9-7-25(8-10-26,15-36-26)30-12-17-2-4-21-24(32-17)33-22(34)14-35-21/h1-4,13,30H,5-10,12,14-15H2,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082389

(CHEMBL3422963)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1Cl |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H28ClN5O4/c1-34-24-18(27)12-19-22(32-24)16(5-11-28-19)4-6-26-9-7-25(8-10-26,15-36-26)29-13-17-2-3-20-23(30-17)31-21(33)14-35-20/h2-3,5,11-12,29H,4,6-10,13-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082428
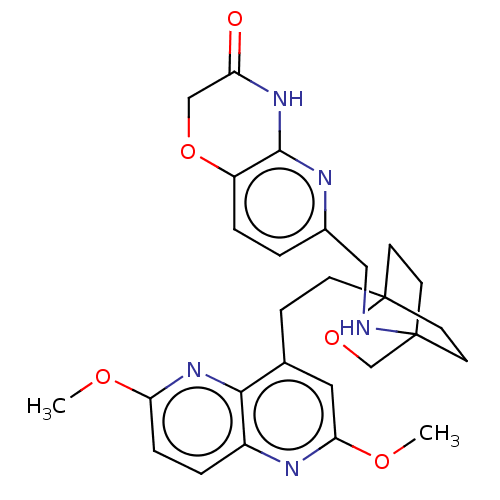
(CHEMBL3422969)Show SMILES COc1ccc2nc(OC)cc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;4.01,-1.54,;5.07,-.92,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H31N5O5/c1-34-22-6-4-19-24(32-22)17(13-23(30-19)35-2)7-8-27-11-9-26(10-12-27,16-37-27)28-14-18-3-5-20-25(29-18)31-21(33)15-36-20/h3-6,13,28H,7-12,14-16H2,1-2H3,(H,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272607
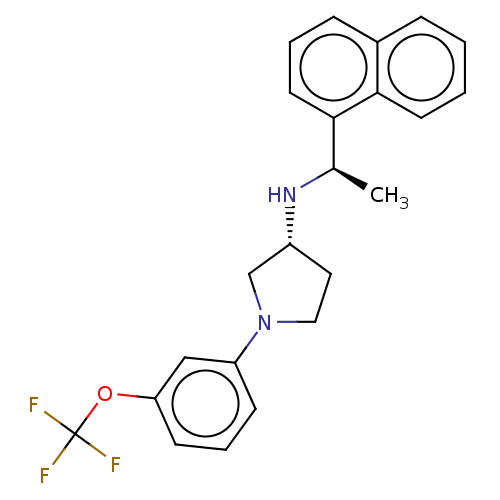
(CHEMBL4126057)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCN(C1)c1cccc(OC(F)(F)F)c1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2O.2ClH/c1-16(21-11-4-7-17-6-2-3-10-22(17)21)27-18-12-13-28(15-18)19-8-5-9-20(14-19)29-23(24,25)26;;/h2-11,14,16,18,27H,12-13,15H2,1H3;2*1H/t16-,18-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082332
(CHEMBL3422765)Show SMILES Oc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-3.75,1.39,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H26FN5O4/c26-17-12-27-18-2-4-20(32)30-22(18)16(17)5-6-25-9-7-24(8-10-25,14-35-25)28-11-15-1-3-19-23(29-15)31-21(33)13-34-19/h1-4,12,28H,5-11,13-14H2,(H,30,32)(H,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data