

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50022633 (CHEMBL3142281 | [1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022635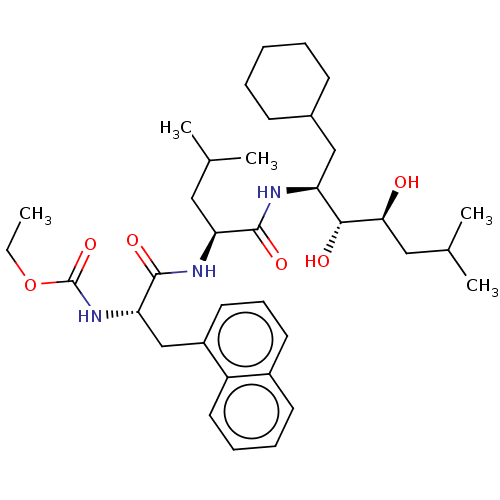 (CHEMBL3142255 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022647 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022646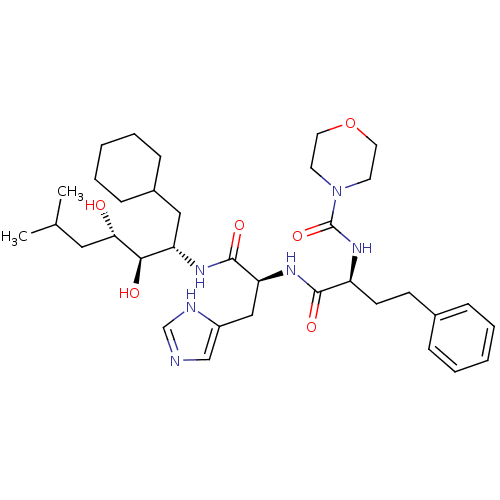 (CHEMBL307917 | Morpholine-4-carboxylic acid {1-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022619 (CHEMBL3348544 | N-[1-(1-Cyclohexylmethyl-2,3-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022597 (CHEMBL3142268 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022650 (CHEMBL75117 | Morpholine-4-carboxylic acid {1-[1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022643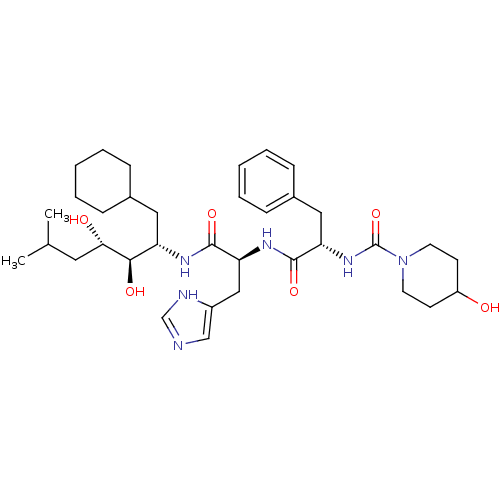 (4-Hydroxy-piperidine-1-carboxylic acid {1-[1-(1-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022649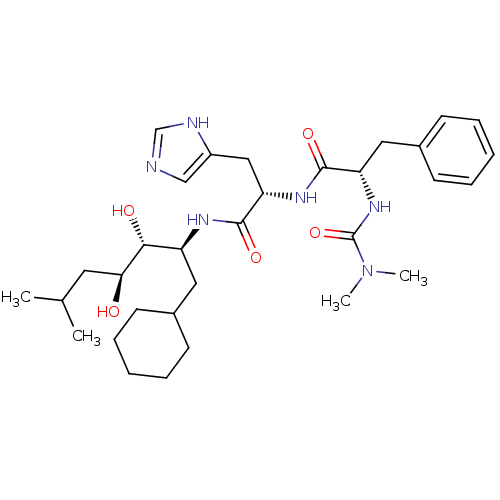 (CHEMBL76458 | N-[1-(1-Cyclohexylmethyl-2,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022628 (CHEMBL3142261 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022603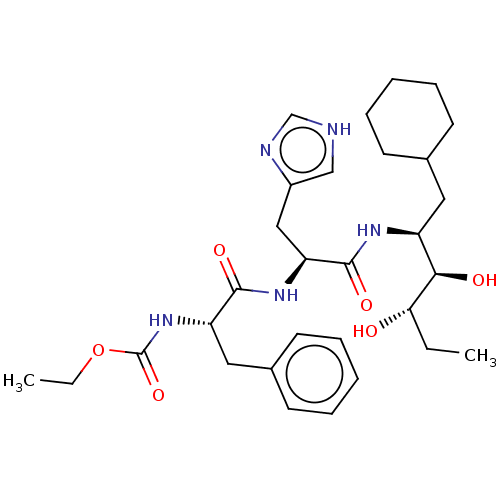 (CHEMBL3348551 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022599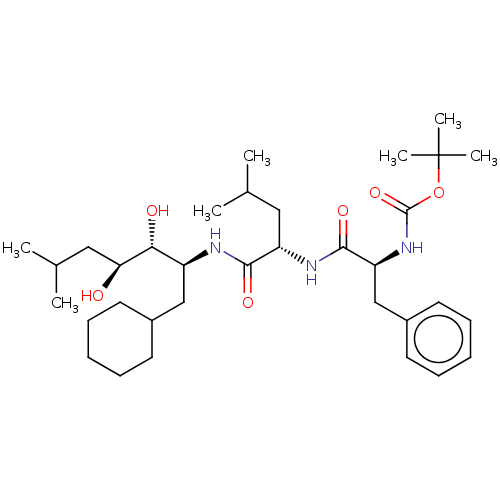 (CHEMBL3142262 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022645 (CHEMBL75510 | N-[1-(1-Cyclohexylmethyl-2,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022651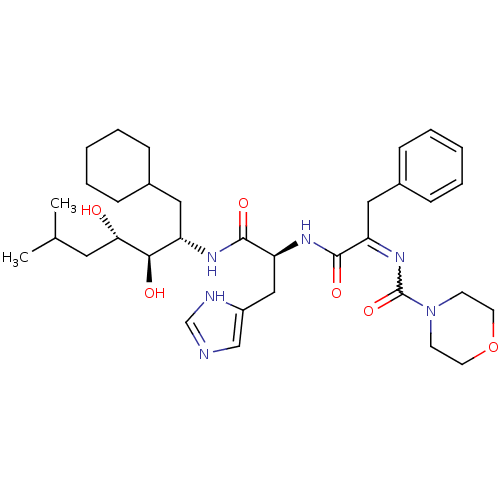 (CHEMBL306274 | Morpholine-4-carboxylic acid {1-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022595 (CHEMBL3348552 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022598 (CHEMBL3348548 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022644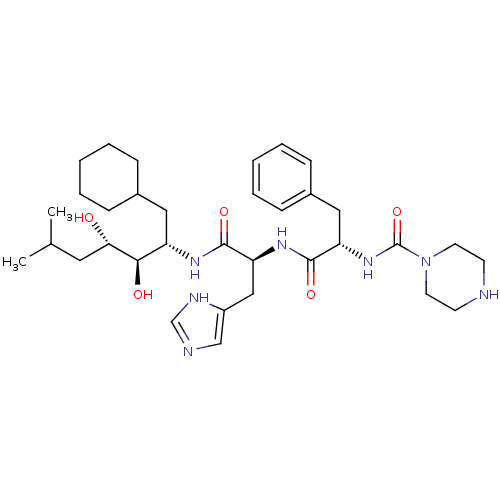 (CHEMBL73712 | Piperazine-1-carboxylic acid {1-[1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma renin (pH 7.4) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022643 (4-Hydroxy-piperidine-1-carboxylic acid {1-[1-(1-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50405479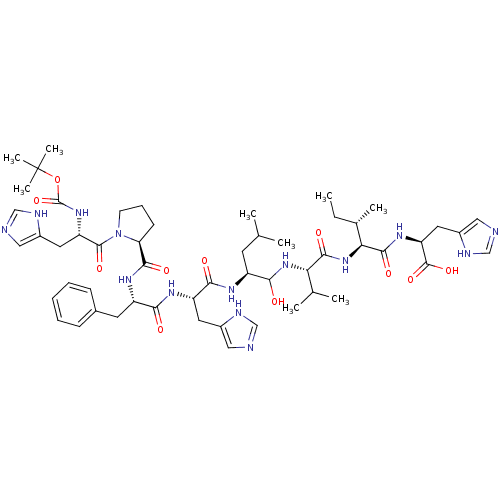 (CHEMBL2028988) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition against human plasma renin | J Med Chem 30: 1729-37 (1987) BindingDB Entry DOI: 10.7270/Q2MK6BW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022630 (CHEMBL3348531 | Cyclohexanecarboxylic acid {1-[1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022637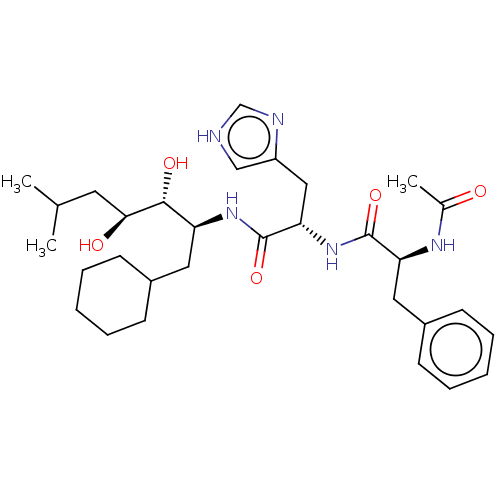 (2-(2-Acetylamino-3-phenyl-propionylamino)-N-(1-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022653 (CHEMBL309345 | Morpholine-4-carboxylic acid [1-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022641 (2-Benzylidene-N-[1-(1-cyclohexylmethyl-2,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022654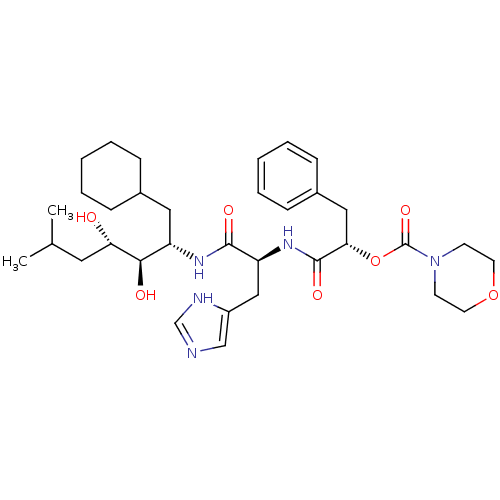 (CHEMBL265485 | Morpholine-4-carboxylic acid 1-[1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma renin (pH 7.4) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022650 (CHEMBL75117 | Morpholine-4-carboxylic acid {1-[1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma renin (pH 7.4) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006189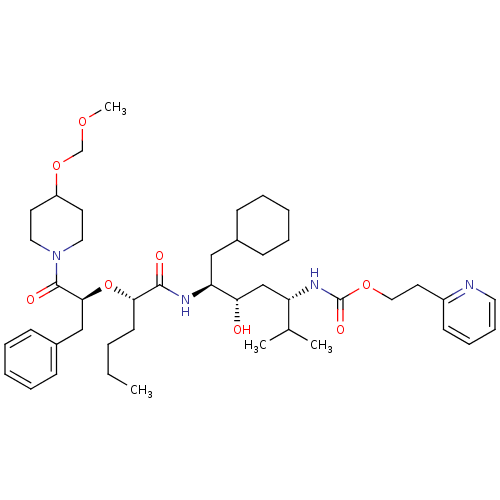 ((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006147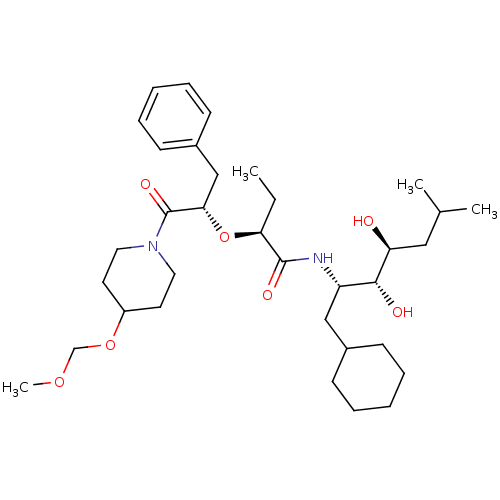 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against purified renin at a pH of 6.0. | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006157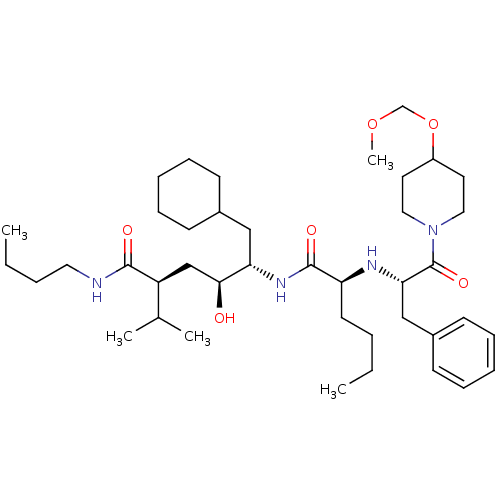 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022642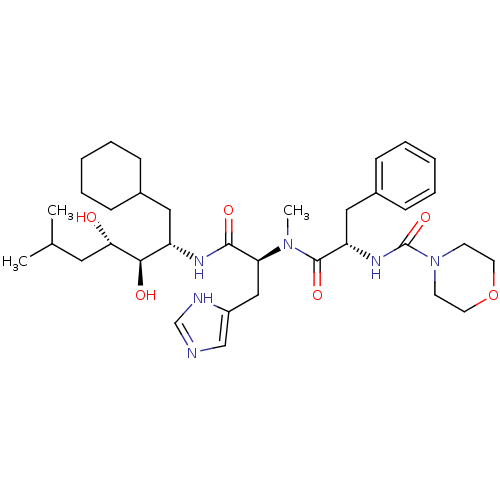 (CHEMBL74765 | Morpholine-4-carboxylic acid (1-{[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma renin (pH 7.4) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022645 (CHEMBL75510 | N-[1-(1-Cyclohexylmethyl-2,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma renin (pH 7.4) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022615 (CHEMBL3348546 | N-{1-[1-(1-Cyclohexylmethyl-2,3-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006185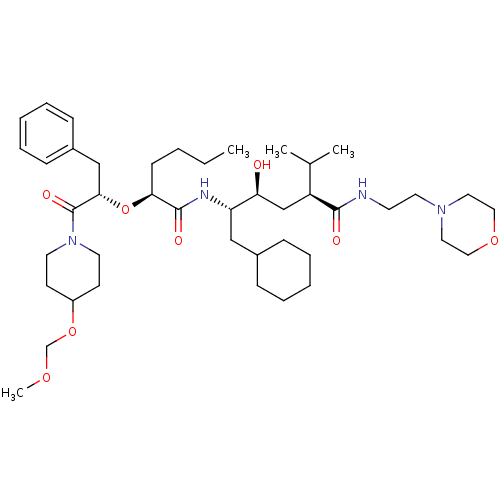 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022611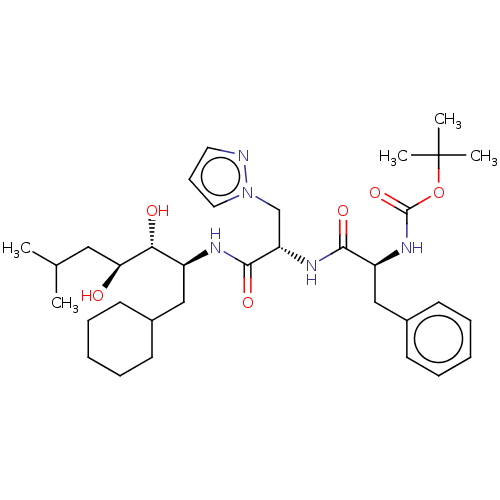 (CHEMBL3142260 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022607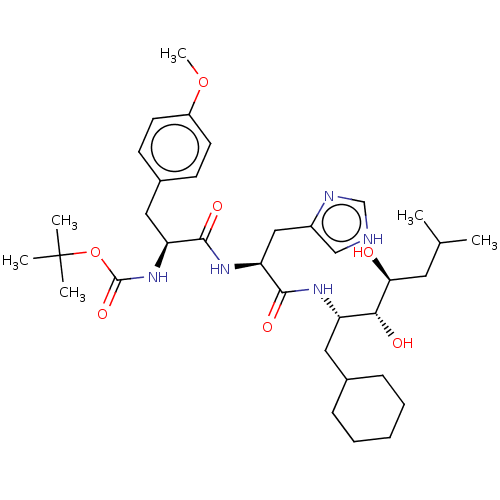 (CHEMBL3348540 | [1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022593 (CHEMBL3142304 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006152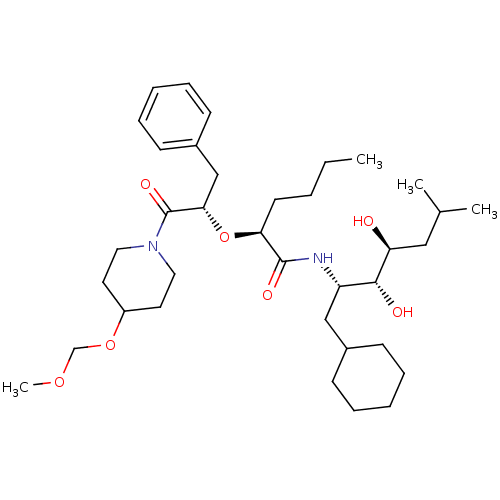 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022638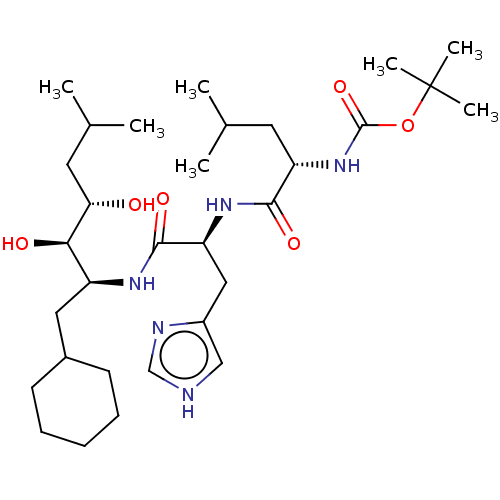 (CHEMBL3348542 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022620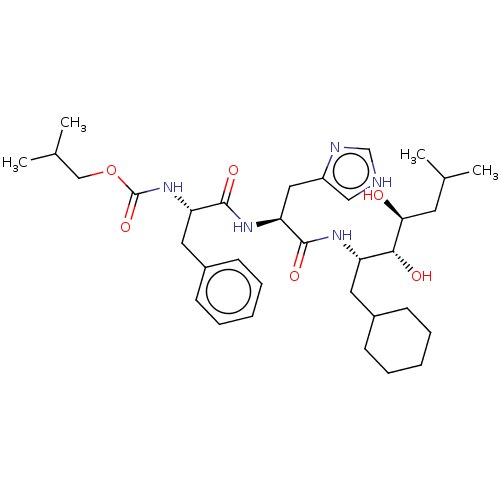 (CHEMBL3348532 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006152 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006206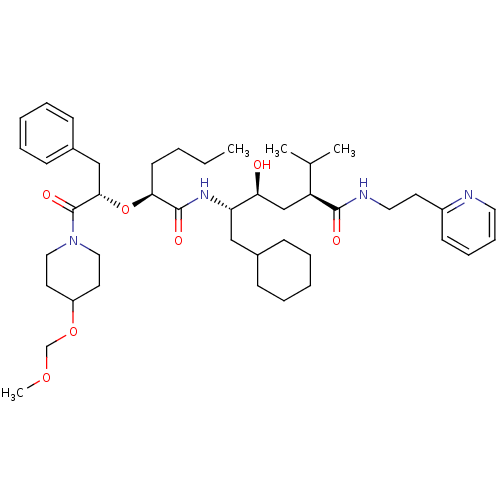 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036999 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006161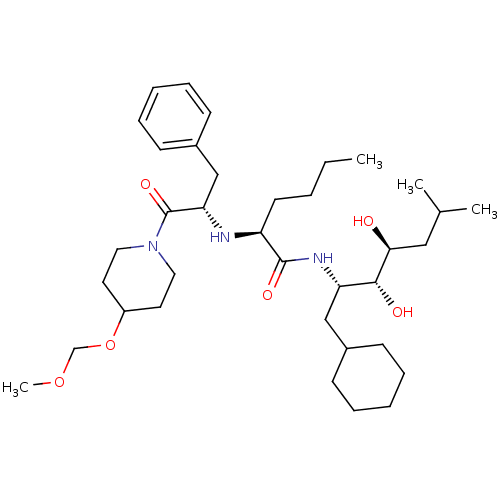 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against purified renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006161 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022636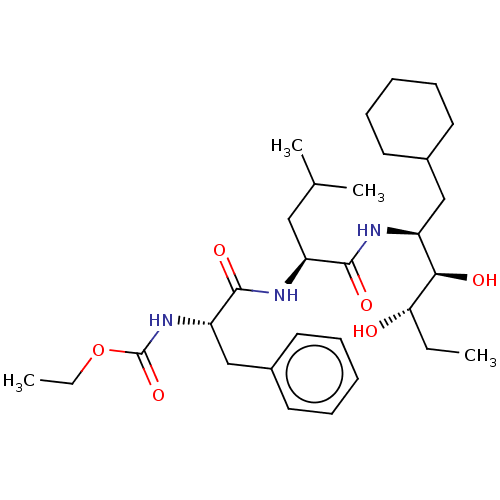 (CHEMBL3142269 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006148 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against purified renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006211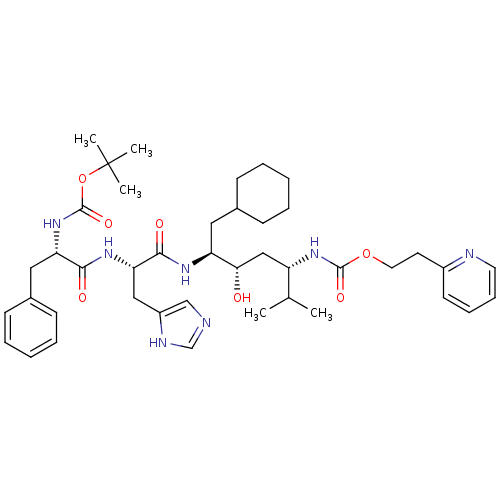 (CHEMBL49218 | {4-[2-(2-tert-Butoxycarbonylamino-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006150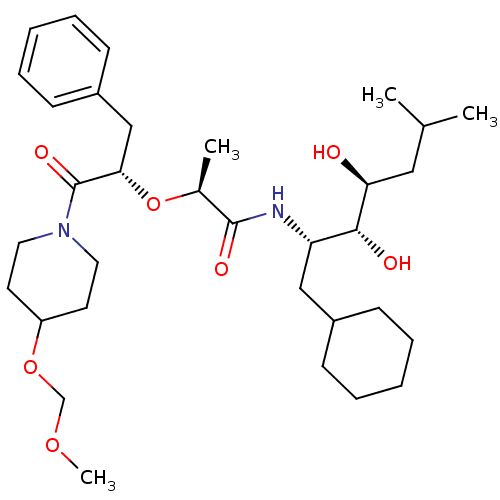 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022647 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036995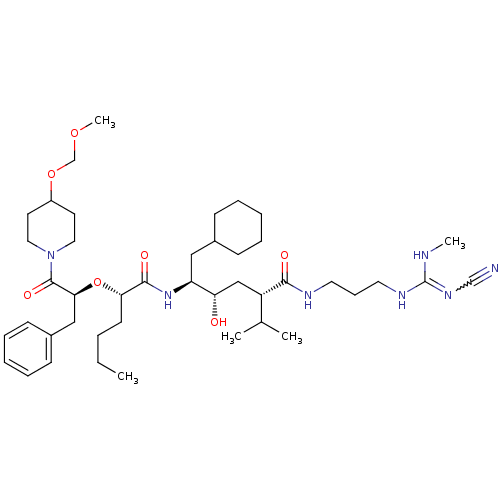 (1N-[3-methylamino(cyano imino)methylaminopropyl]-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006204 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 482 total ) | Next | Last >> |