

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50444455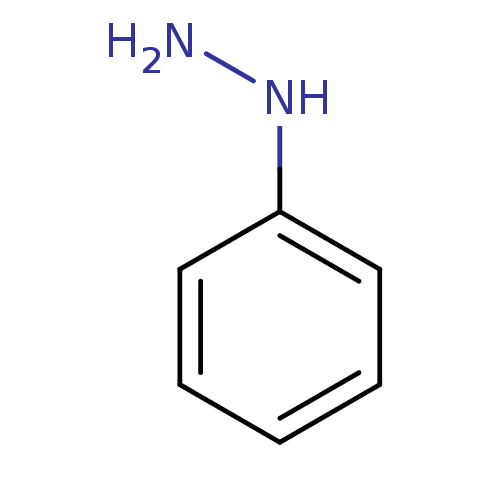 (CHEBI:27924 | Phenylhydrazine) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50444455 (CHEBI:27924 | Phenylhydrazine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50444455 (CHEBI:27924 | Phenylhydrazine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50444457 (CHEMBL3092383) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50444456 (2-Hydrazinylbenzo[D]Thiazole | CHEMBL1933308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50444456 (2-Hydrazinylbenzo[D]Thiazole | CHEMBL1933308) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50444456 (2-Hydrazinylbenzo[D]Thiazole | CHEMBL1933308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50444459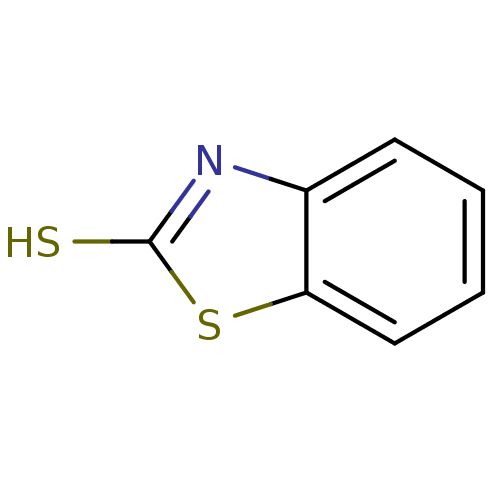 (2-Mercaptobenzothiazole | 2-Mercaptobenzothioazole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50444458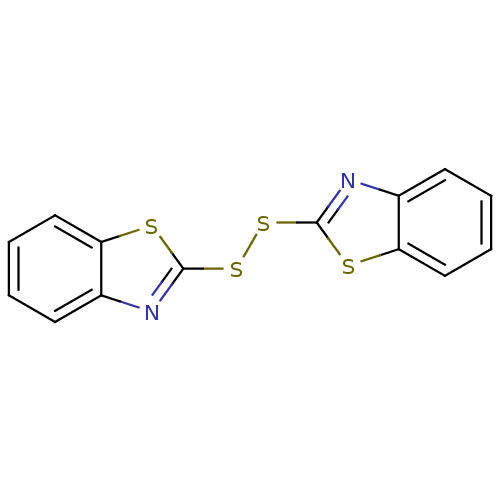 (BENZOTHIAZYL DISULFIDE | BI-87F4 | CHEBI:53239) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50335098 (6-Nitro-benzothiazol-2-ylamine | 6-nitrobenzo[d]th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50444460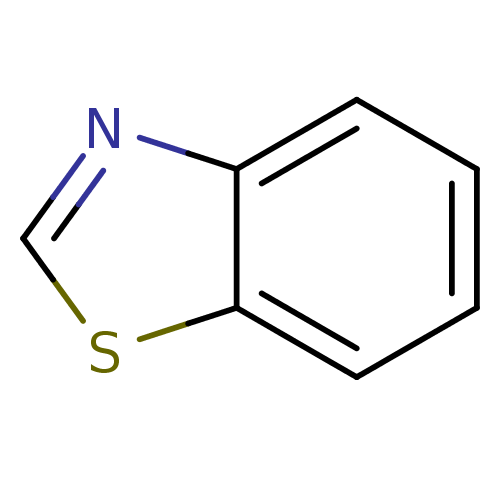 (Benzo[D]Thiazole | Benzothiazole | CHEBI:45993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr | Bioorg Med Chem 21: 7595-603 (2013) Article DOI: 10.1016/j.bmc.2013.10.037 BindingDB Entry DOI: 10.7270/Q2377B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||