

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM50370291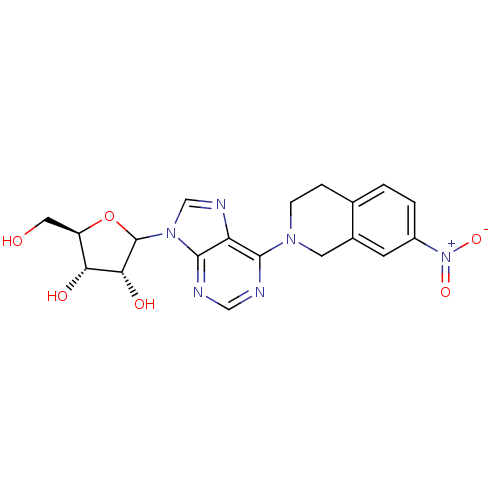 (CHEMBL608208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center Curated by ChEMBL | Assay Description Binding affinity of compound towards Nucleoside transporter es-type by facile competitive binding flow cytometric assay using the human K562 chronic ... | J Med Chem 46: 831-7 (2003) Article DOI: 10.1021/jm020405p BindingDB Entry DOI: 10.7270/Q21R6R8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM23617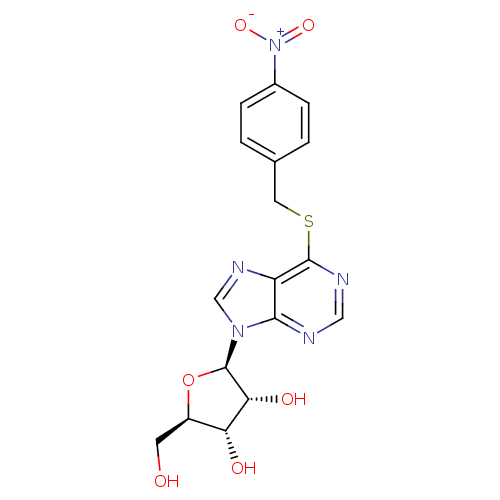 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-{[(4-nitrophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center Curated by ChEMBL | Assay Description Binding affinity of compound towards Nucleoside transporter es-type by facile competitive binding flow cytometric assay using the human K562 chronic ... | J Med Chem 46: 831-7 (2003) Article DOI: 10.1021/jm020405p BindingDB Entry DOI: 10.7270/Q21R6R8G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM50370288 (CHEMBL607631) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center Curated by ChEMBL | Assay Description Binding affinity of compound towards Nucleoside transporter es-type by facile competitive binding flow cytometric assay using the human K562 chronic ... | J Med Chem 46: 831-7 (2003) Article DOI: 10.1021/jm020405p BindingDB Entry DOI: 10.7270/Q21R6R8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM50370292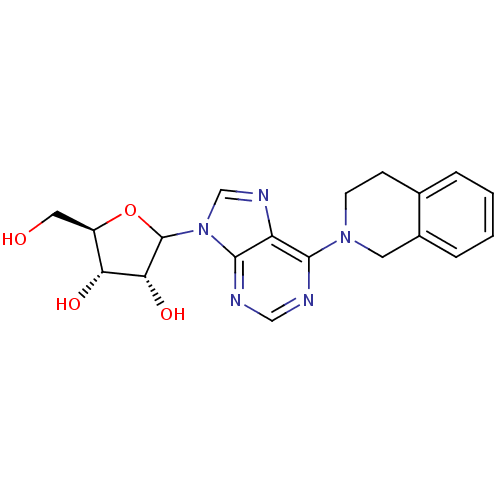 (CHEMBL608205) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center Curated by ChEMBL | Assay Description Binding affinity of compound towards Nucleoside transporter es-type by facile competitive binding flow cytometric assay using the human K562 chronic ... | J Med Chem 46: 831-7 (2003) Article DOI: 10.1021/jm020405p BindingDB Entry DOI: 10.7270/Q21R6R8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM50370289 (CHEMBL608481) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center Curated by ChEMBL | Assay Description Binding affinity of compound towards Nucleoside transporter es-type by facile competitive binding flow cytometric assay using the human K562 chronic ... | J Med Chem 46: 831-7 (2003) Article DOI: 10.1021/jm020405p BindingDB Entry DOI: 10.7270/Q21R6R8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM50370290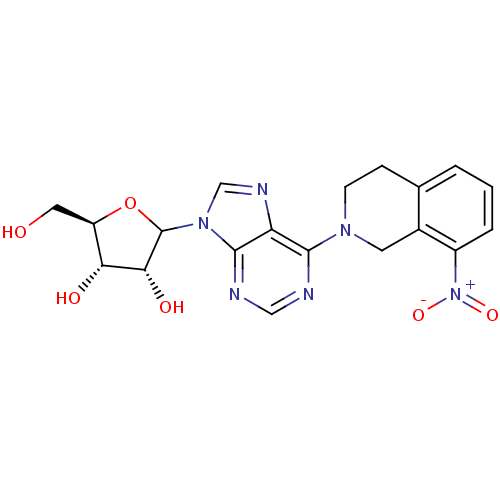 (CHEMBL608227) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center Curated by ChEMBL | Assay Description Binding affinity of compound towards Nucleoside transporter es-type by facile competitive binding flow cytometric assay using the human K562 chronic ... | J Med Chem 46: 831-7 (2003) Article DOI: 10.1021/jm020405p BindingDB Entry DOI: 10.7270/Q21R6R8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||