

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase TEM (Escherichia coli) | BDBM50284790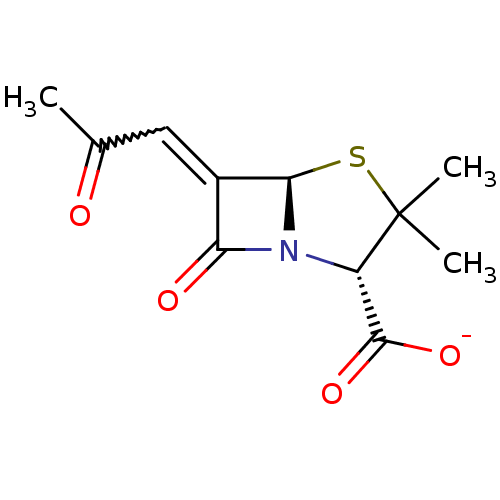 (CHEMBL35987 | Sodium; (2S,5R)-3,3-dimethyl-7-oxo-6...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Escherichia coli WC3310 TEM-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50033680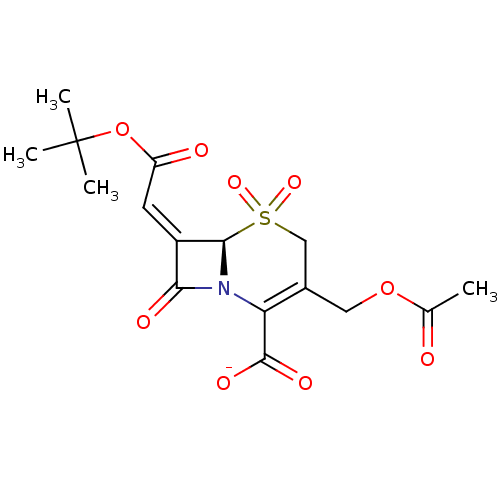 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Escherichia coli WC3310 TEM-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50284783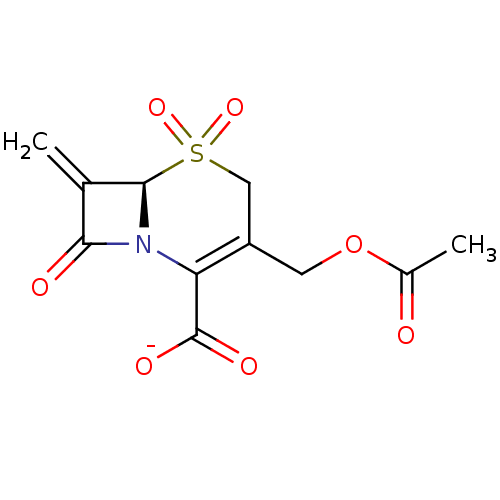 (CHEMBL37794 | Sodium; (R)-3-acetoxymethyl-7-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Enterobacter cloacae SC 12368 E-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Escherichia coli WC3310 TEM-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50284783 (CHEMBL37794 | Sodium; (R)-3-acetoxymethyl-7-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Enterobacter cloacae P99 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50284786 (CHEMBL36657 | Sodium; (R)-3-acetoxymethyl-7-[1-met...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Escherichia coli WC3310 TEM-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM350085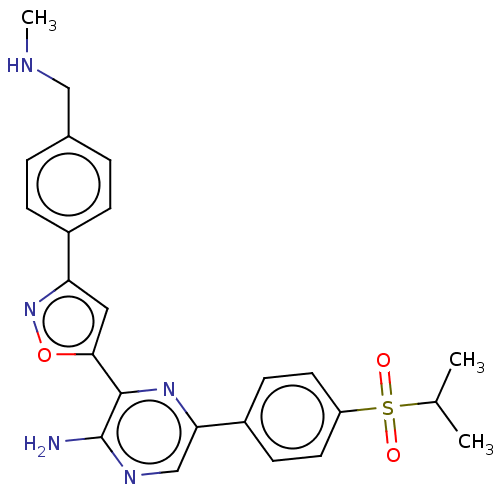 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50284789 ((2S,5R,6Z)-6-(carboxymethylene)-3,3-dimethyl-7-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Enterobacter cloacae P99 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Escherichia coli WC3310 TEM-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50284789 ((2S,5R,6Z)-6-(carboxymethylene)-3,3-dimethyl-7-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Enterobacter cloacae SC 12368 E-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50284789 ((2S,5R,6Z)-6-(carboxymethylene)-3,3-dimethyl-7-oxo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Escherichia coli WC3310 TEM-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50284794 (CHEMBL36689 | Sodium; (R)-3-acetoxymethyl-5,5,8-tr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Enterobacter cloacae P99 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50284793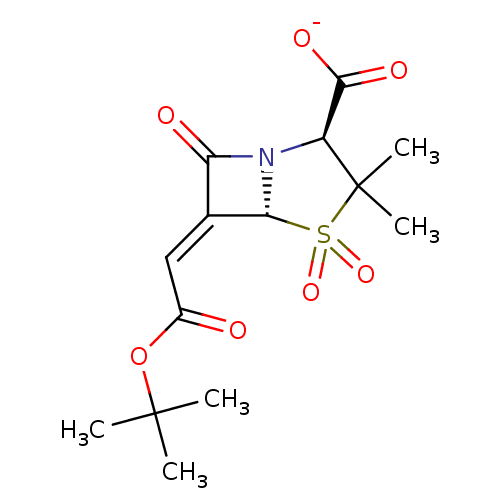 (CHEMBL284539 | Sodium; (2S,5R)-6-[1-tert-butoxycar...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Escherichia coli WC3310 TEM-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50284784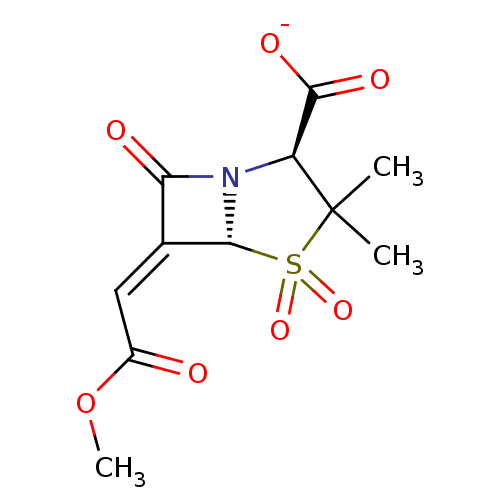 (CHEMBL290112 | Sodium; (2S,5R)-6-[1-methoxycarbony...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50284794 (CHEMBL36689 | Sodium; (R)-3-acetoxymethyl-5,5,8-tr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Enterobacter cloacae SC 12368 E-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50284784 (CHEMBL290112 | Sodium; (2S,5R)-6-[1-methoxycarbony...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 308 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Escherichia coli WC3310 TEM-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50284790 (CHEMBL35987 | Sodium; (2S,5R)-3,3-dimethyl-7-oxo-6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 361 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Enterobacter cloacae P99 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50284792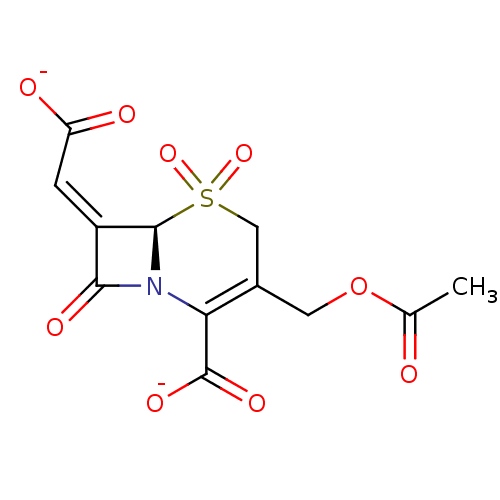 ((6R,7Z)-3-[(acetyloxy)methyl]-7-(2-oxido-2-oxoethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Escherichia coli WC3310 TEM-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Helicobacter pylori J99) | BDBM50256537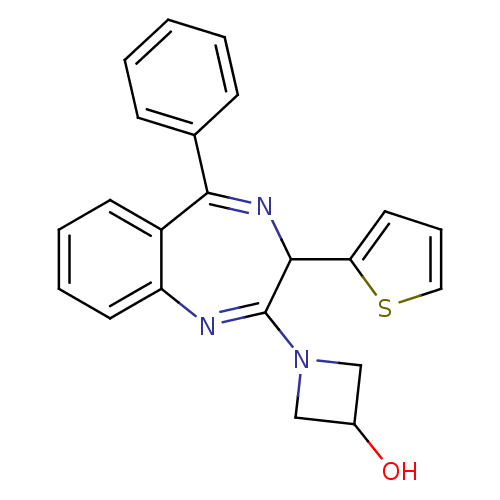 (1-(5-phenyl-3-(thiophen-2-yl)-3H-benzo[e][1,4]diaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of MurI in wild type Helicobacter pylori J99 | Bioorg Med Chem Lett 19: 930-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.113 BindingDB Entry DOI: 10.7270/Q2J38SDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536589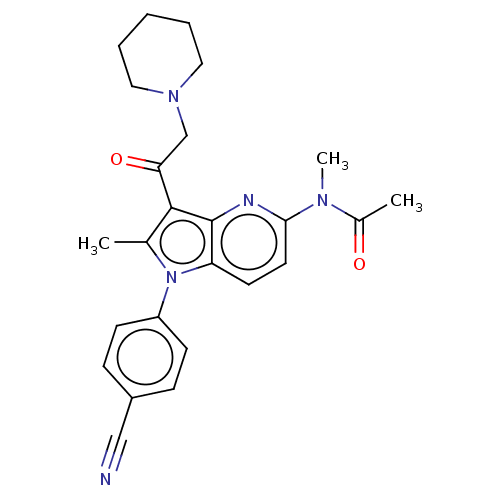 (US11242361, Compound 196) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536590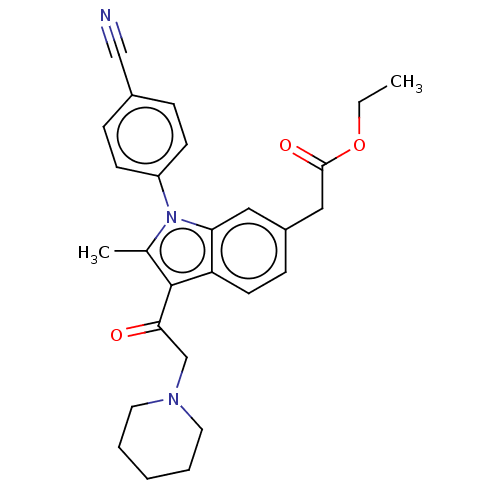 (US11242361, Compound 197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536591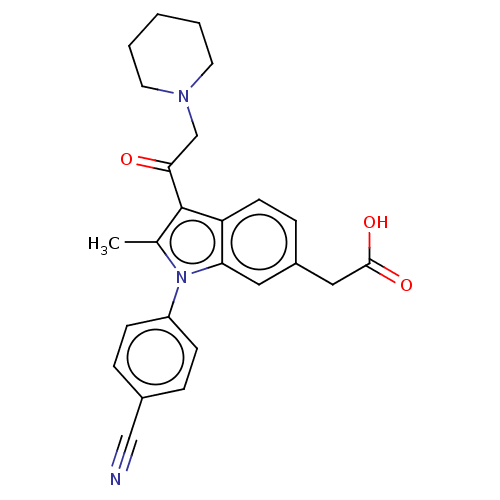 (US11242361, Compound 198) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536592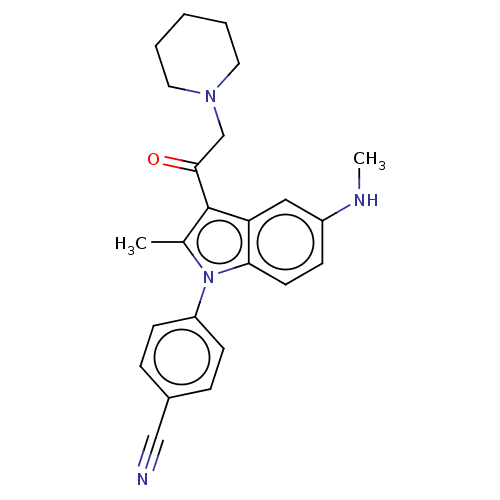 (US11242361, Compound 199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536593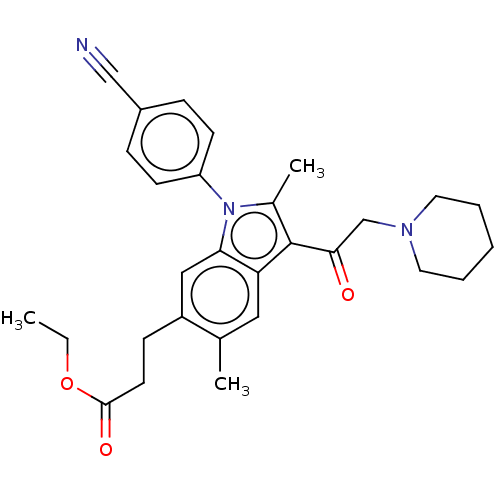 (US11242361, Compound 200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536594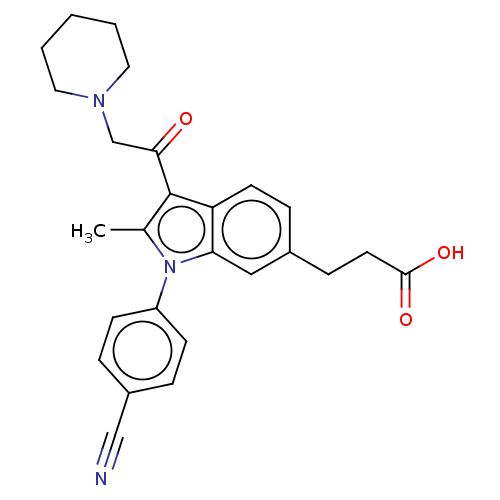 (US11242361, Compound 201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536595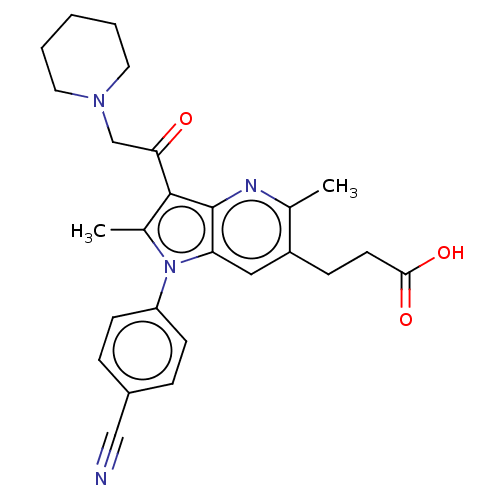 (US11242361, Compound 202) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536596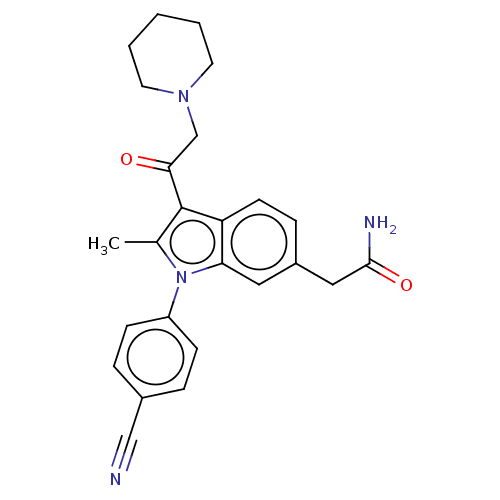 (US11242361, Compound 203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536597 (US11242361, Compound 204) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536598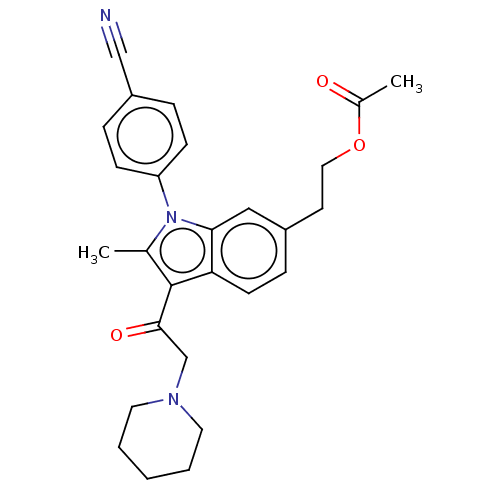 (US11242361, Compound 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536599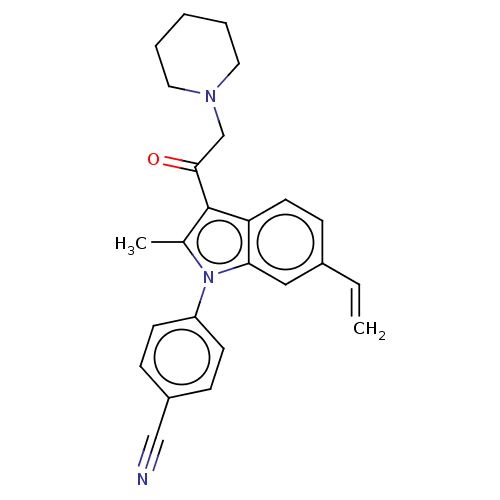 (US11242361, Compound 206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536600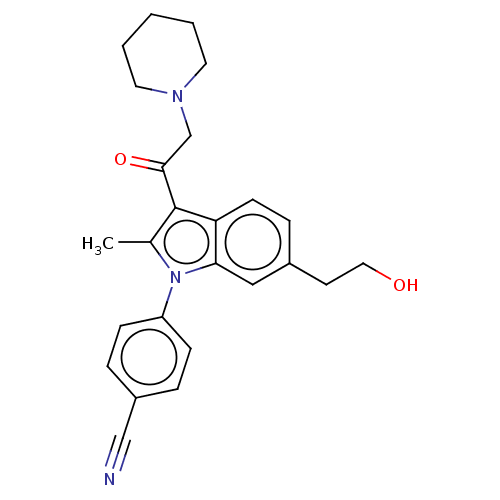 (US11242361, Compound 207) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536601 (US11242361, Compound 208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536602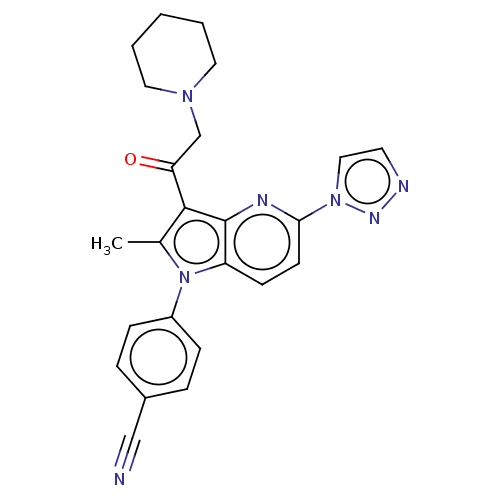 (US11242361, Compound 209) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536603 (US11242361, Compound 210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536604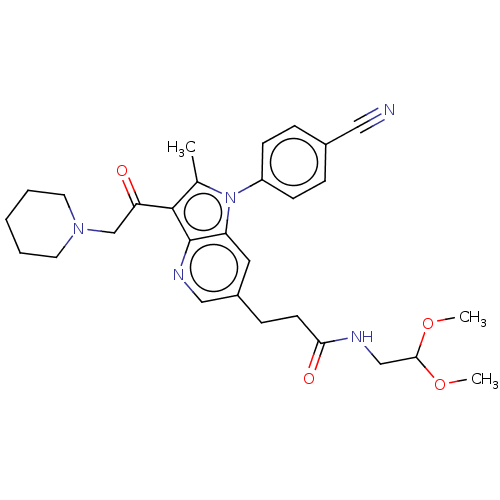 (US11242361, Compound 296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536605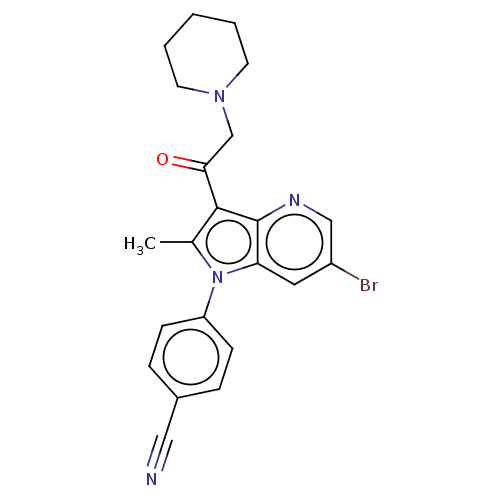 (4-(6-Bromo-2-methyl-3-(2-(piperidin-1-yl)acetyl)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536606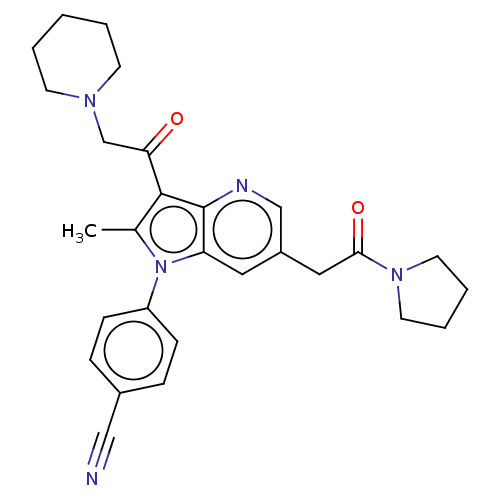 (US11242361, Compound 298) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536607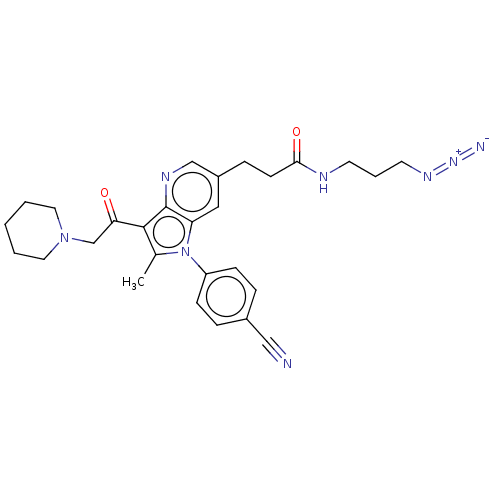 (US11242361, Compound 299) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536608 (US11242361, Compound 300) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536609 (US11242361, Compound 301) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536610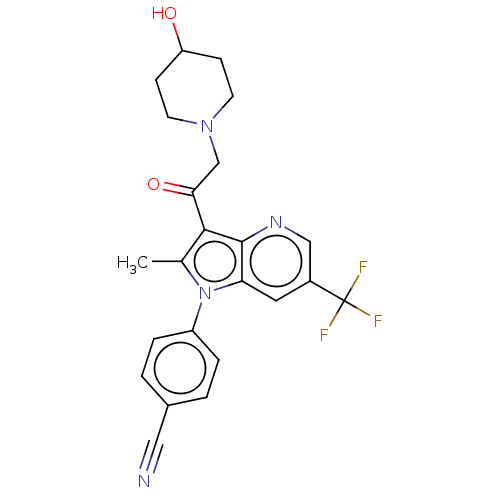 (US11242361, Compound 302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536611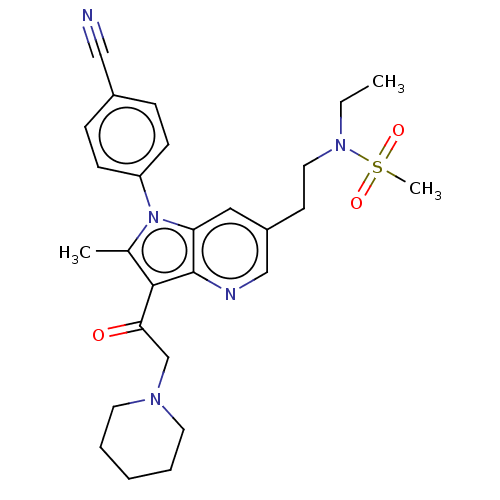 (US11242361, Compound 303) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536612 (US11242361, Compound 304) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536613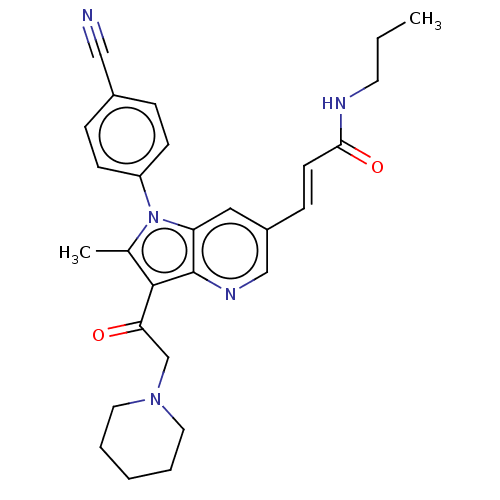 (US11242361, Compound 305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536614 (US11242361, Compound 306) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536615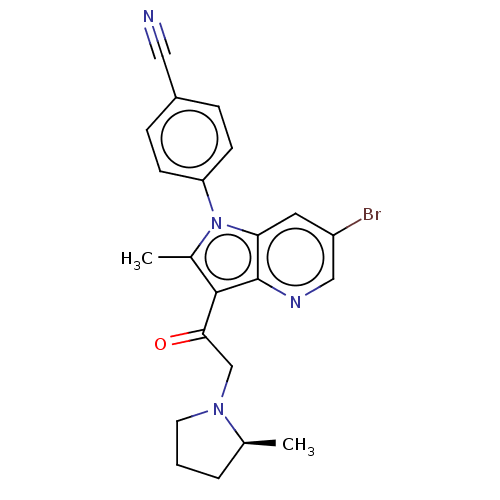 (US11242361, Compound 307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536616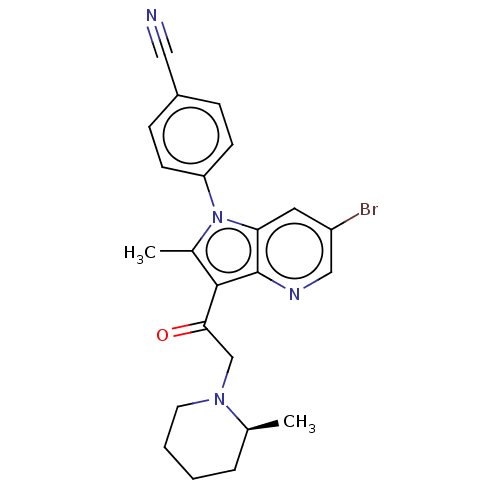 (US11242361, Compound 308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536617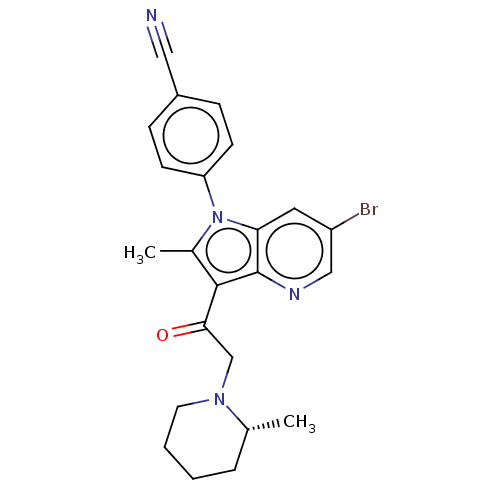 (US11242361, Compound 309) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536618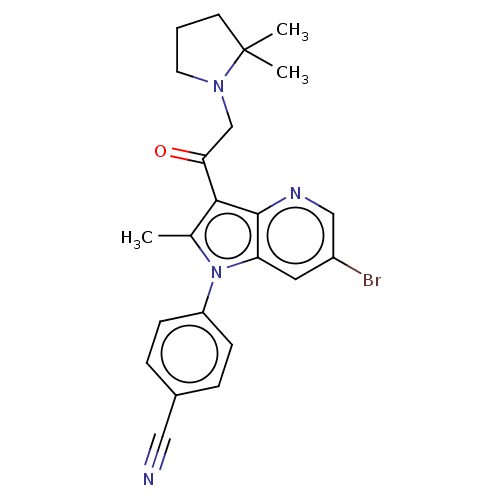 (US11242361, Compound 310) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM536619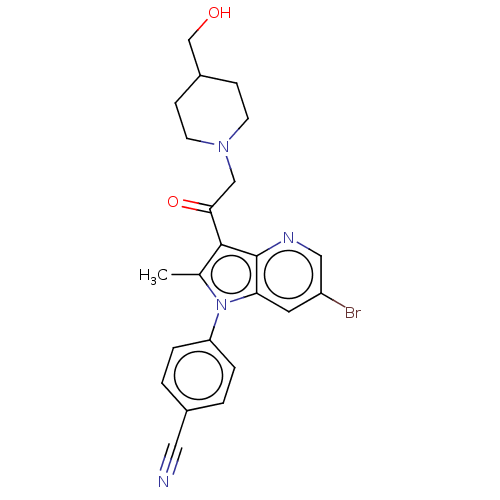 (US11242361, Compound 311 | US11242361, Compound 31...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using previously described methodology [B. H. Lee et al. Nature 2010, 467 (9), 179, the contents of which are expressly incorporated by reference her... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 468 total ) | Next | Last >> |