

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50033680 CHEMBL268919::Sodium; (R)-3-acetoxymethyl-7-[1-tert-butoxycarbonyl-meth-(Z)-ylidene]-5,5,8-trioxo-5lambda*6*-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate
SMILES: CC(=O)OCC1=C(N2[C@@H](\C(=C/C(=O)OC(C)(C)C)C2=O)S(=O)(=O)C1)C([O-])=O
InChI Key: InChIKey=LQVZDTYWLLBGHZ-XTXQVDMPSA-M
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Enterobacter cloacae) | BDBM50033680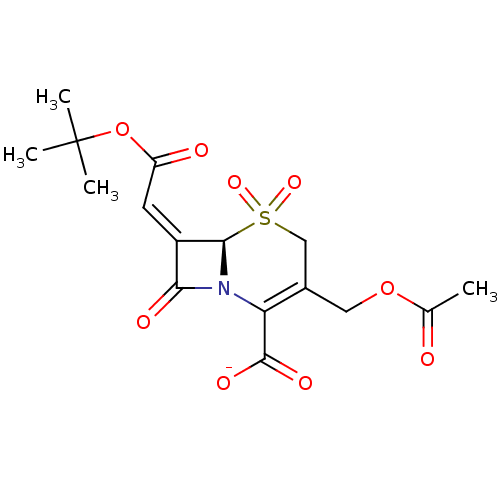 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Enterobacter cloacae P99 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50033680 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (TEM-1) (Escherichia coli) | BDBM50033680 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The concentration of compound to inhibit beta-lactamase was measured on E. coli WC3310 | J Med Chem 38: 1022-34 (1995) BindingDB Entry DOI: 10.7270/Q29S1Q29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50033680 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The concentration of compound to inhibit beta-lactamase was measured on Enterobacter cloacae P99 | J Med Chem 38: 1022-34 (1995) BindingDB Entry DOI: 10.7270/Q29S1Q29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50033680 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase class C (Enterobacter cloacae) | BDBM50033680 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (TEM-1) (Escherichia coli) | BDBM50033680 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against beta-Lactamase enzyme derived from Escherichia coli WC3310 TEM-2 | Bioorg Med Chem Lett 5: 1513-1518 (1995) Article DOI: 10.1016/0960-894X(95)00249-S BindingDB Entry DOI: 10.7270/Q2ZW1MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50033680 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50033680 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The concentration of compound to inhibit beta-lactamase was measured on E. cloacae SC 12368 | J Med Chem 38: 1022-34 (1995) BindingDB Entry DOI: 10.7270/Q29S1Q29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||