

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479471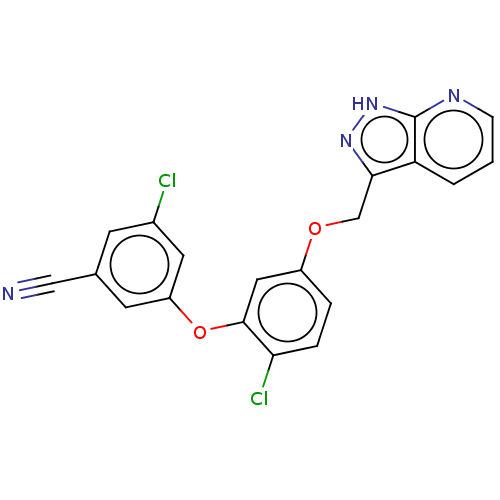 (CHEMBL491019 | MK-1107) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484635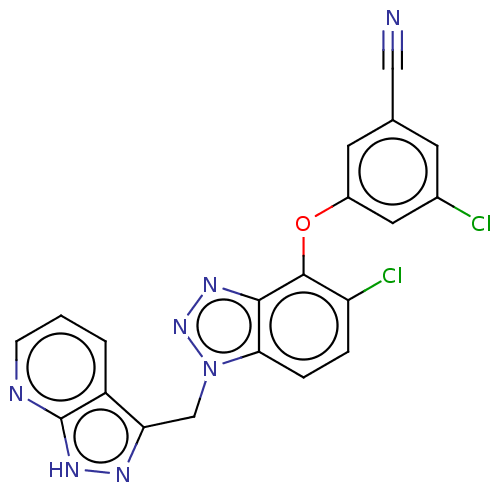 (CHEMBL1939500) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484496 (CHEMBL1928648) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484632 (Mk-6186 | Mk6186) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484629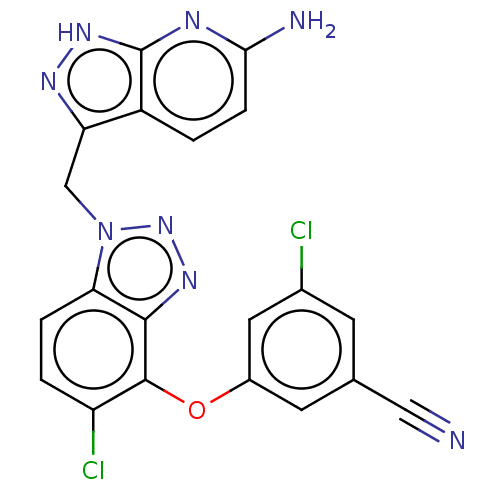 (CHEMBL1939503) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484495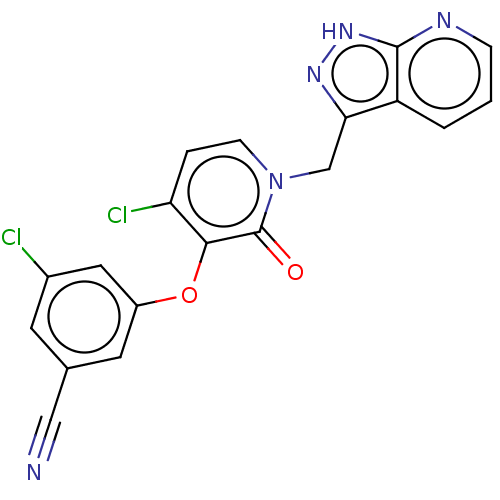 (CHEMBL1928645) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484493 (CHEMBL1928646) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484630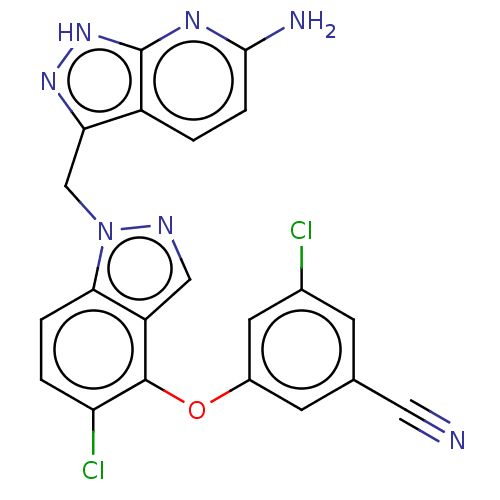 (CHEMBL1939502 | MK-7445) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484494 (CHEMBL1928647) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484492 (CHEMBL1928643) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484624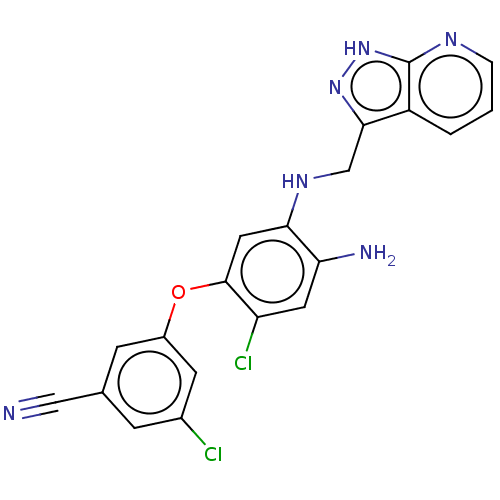 (CHEMBL1939510) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484631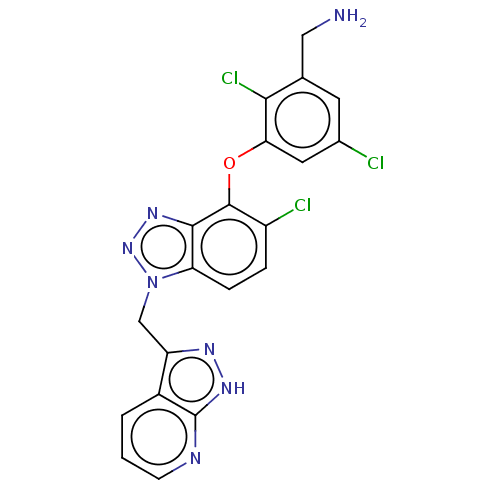 (CHEMBL1939501) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484634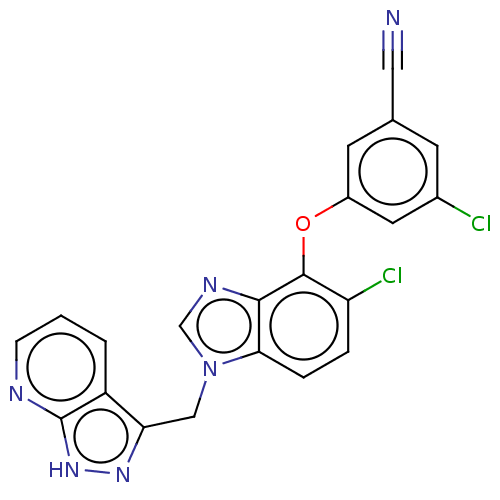 (CHEMBL1939504) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484633 (CHEMBL1939507) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484497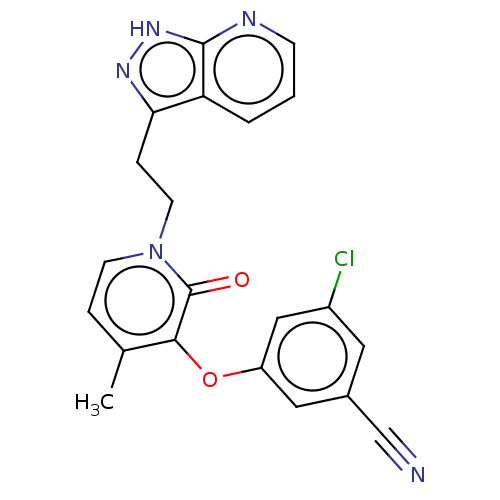 (CHEMBL1928644) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484625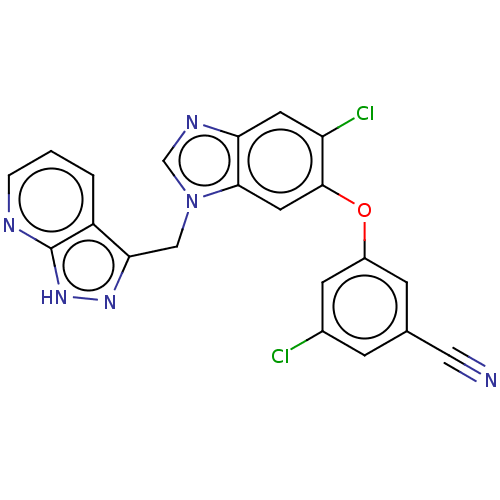 (CHEMBL1939509) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484626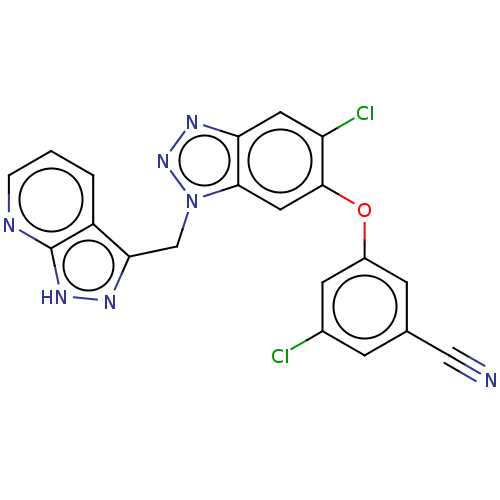 (CHEMBL1939508) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484628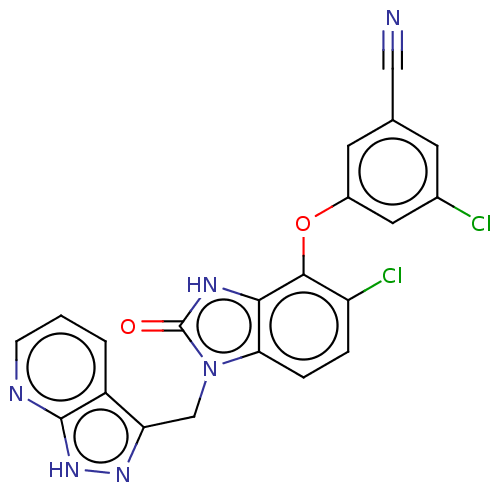 (CHEMBL1939505) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484627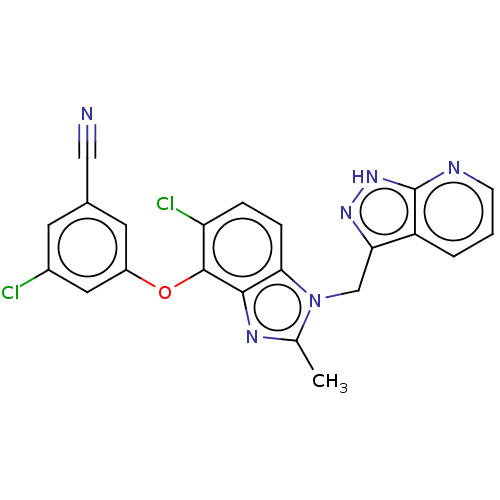 (CHEMBL1939506) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50079961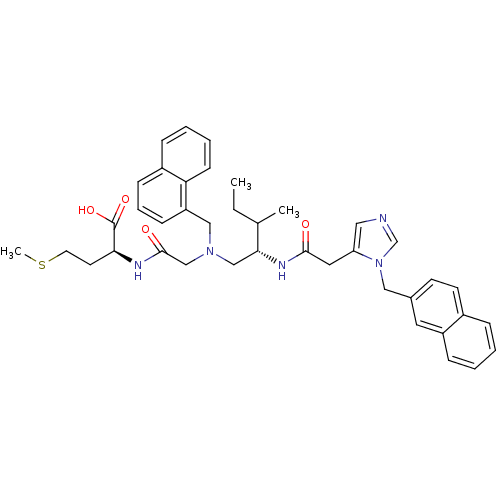 ((S)-2-[2-({(S)-3-Methyl-2-[2-(3-naphthalen-2-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50079974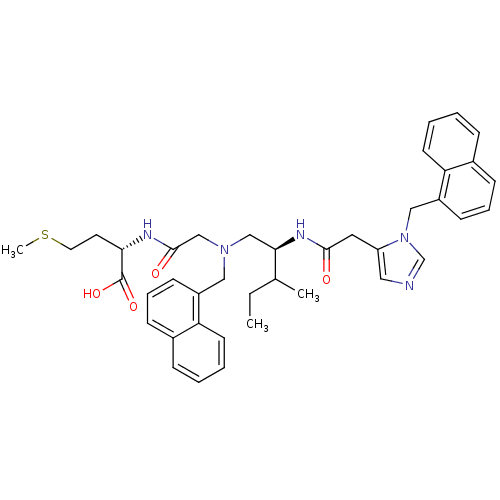 ((S)-2-[2-({(S)-3-Methyl-2-[2-(3-naphthalen-1-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50079956 ((S)-2-[2-({(S)-2-[2-(3-Benzyl-3H-imidazol-4-yl)-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50369443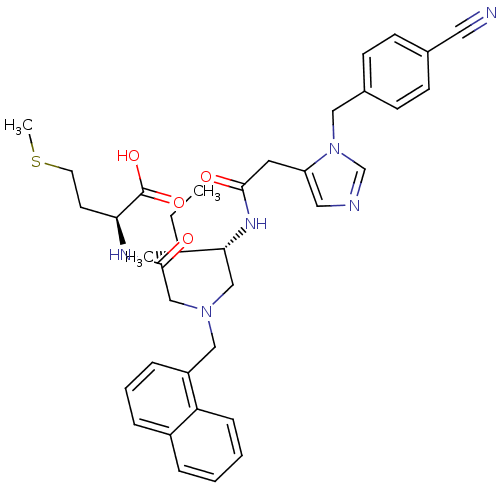 (CHEMBL252953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50079966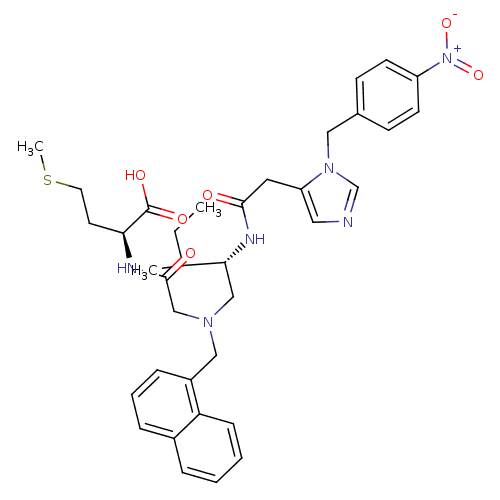 ((S)-2-{2-[((S)-3-Methyl-2-{2-[3-(4-nitro-benzyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50079978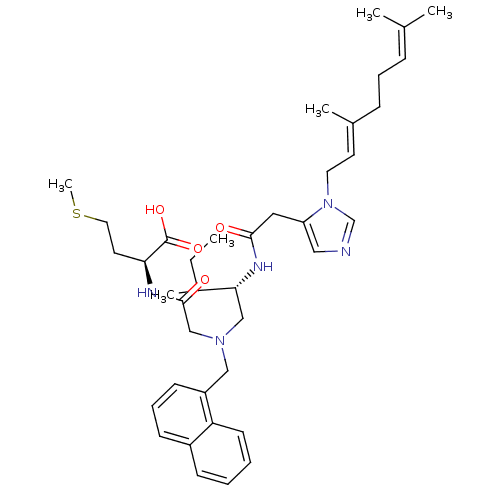 ((S)-2-{2-[((S)-2-{2-[3-((E)-3,7-Dimethyl-octa-2,6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50079971 ((S)-2-{2-[((S)-2-{2-[3-(4-Fluoro-benzyl)-3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50079968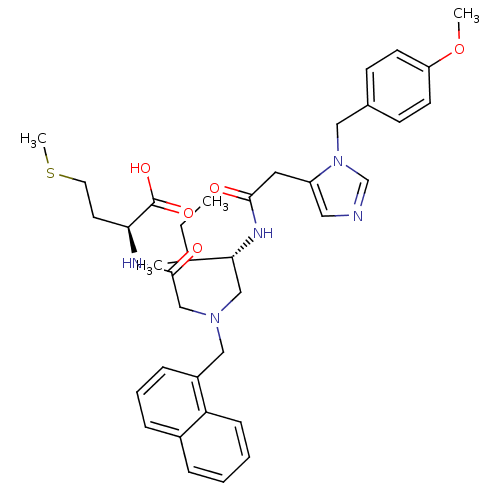 ((S)-2-{2-[((S)-2-{2-[3-(4-Methoxy-benzyl)-3H-imida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50079962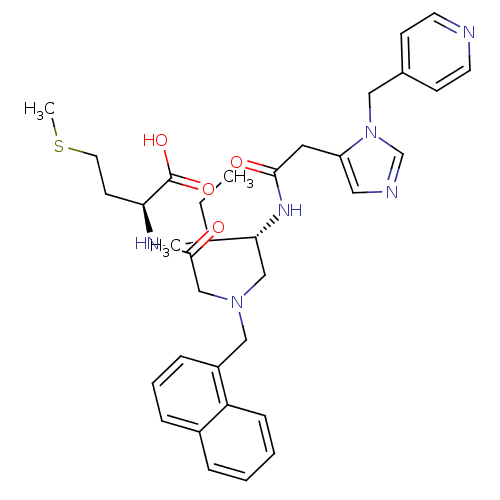 ((S)-2-[2-({(S)-3-Methyl-2-[2-(3-pyridin-4-ylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50079963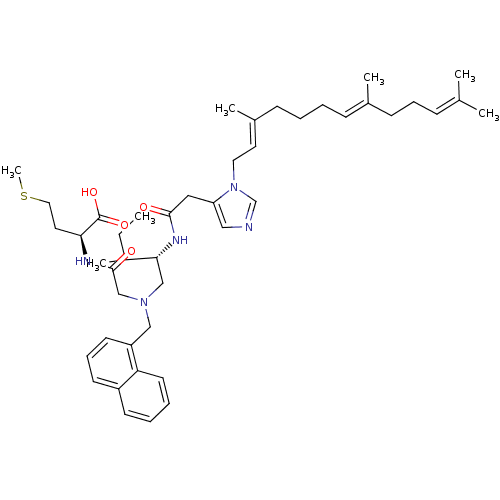 ((S)-4-Methylsulfanyl-2-{2-[((S)-3-methyl-2-{2-[3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560274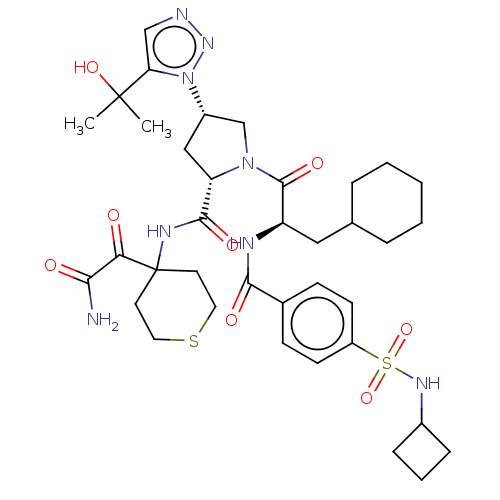 (US11377439, Example 111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50497566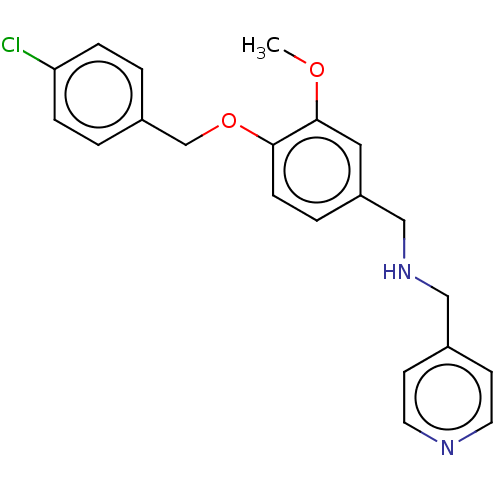 (CHEMBL3344193) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann-Wolfgang-Goethe University of Frankfurt Curated by ChEMBL | Assay Description Inhibition of Plk1 immunoprecipitated from human HeLa cells using casein substrate after 12 to 26 hrs by autoradiography | Bioorg Med Chem Lett 24: 5063-9 (2014) Article DOI: 10.1016/j.bmcl.2014.09.015 BindingDB Entry DOI: 10.7270/Q26Q2171 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560275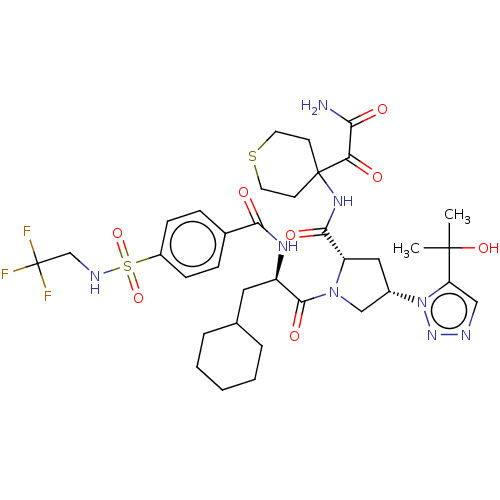 (US11377439, Example 112) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50079964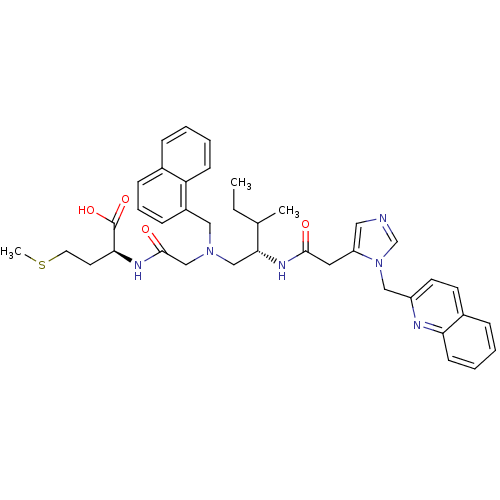 ((S)-2-[2-({(S)-3-Methyl-2-[2-(3-quinolin-2-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase | J Med Chem 42: 3356-68 (1999) Article DOI: 10.1021/jm990080l BindingDB Entry DOI: 10.7270/Q27P9020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560277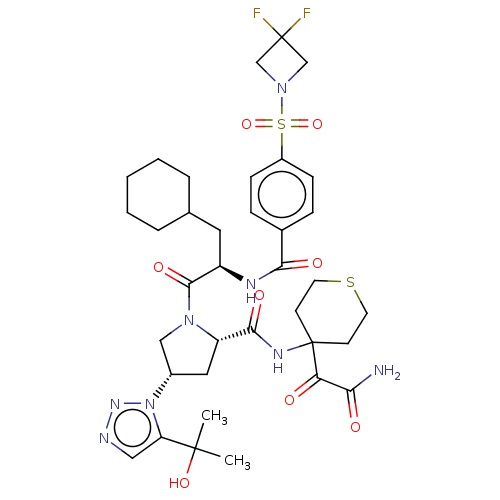 (US11377439, Example 114) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560273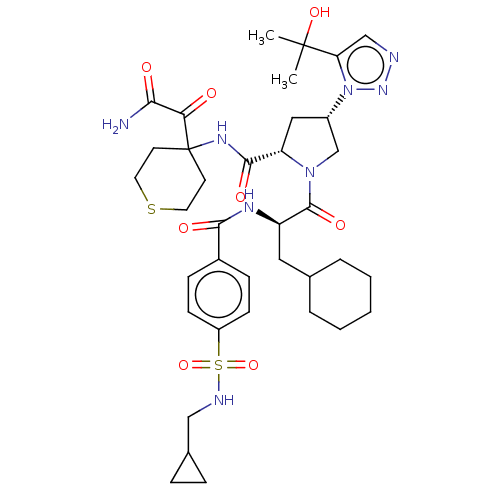 (US11377439, Example 110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560270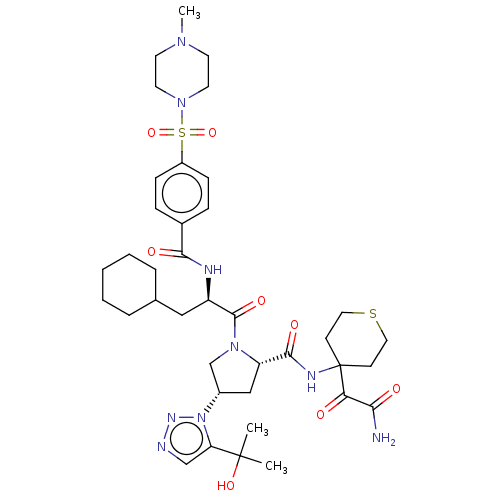 (US11377439, Example 107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560266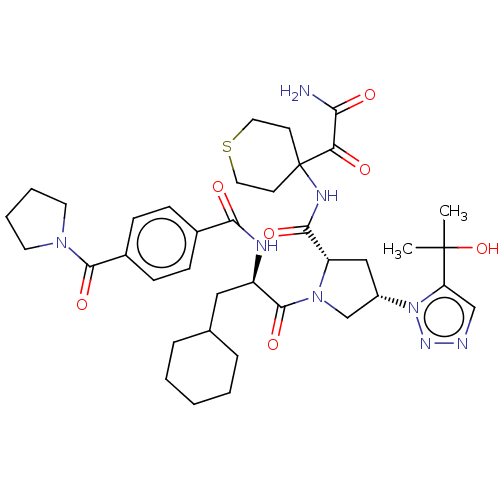 (US11377439, Example 103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560279 (US11377439, Example 116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560251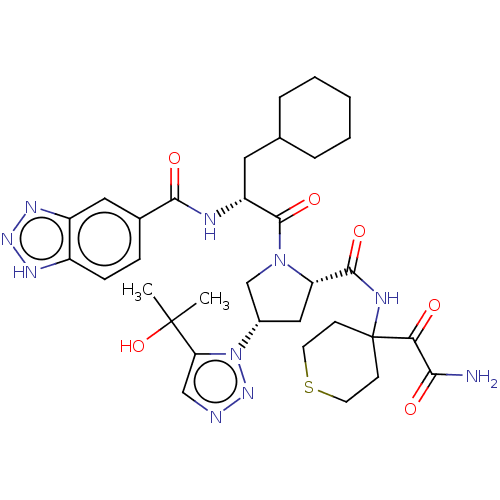 (US11377439, Example 88) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560256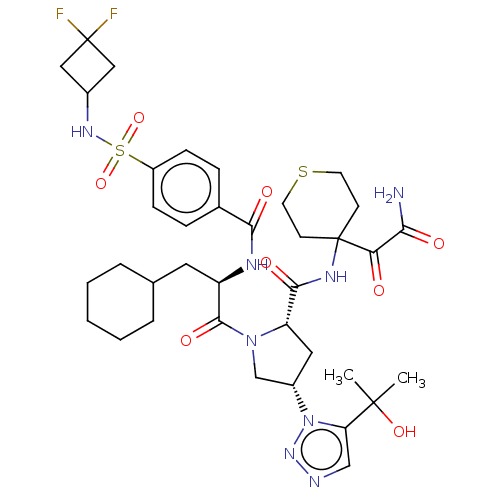 (US11377439, Example 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560238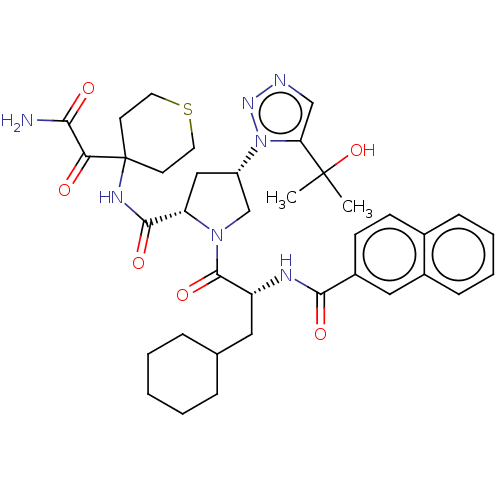 (US11377439, Example 75) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560276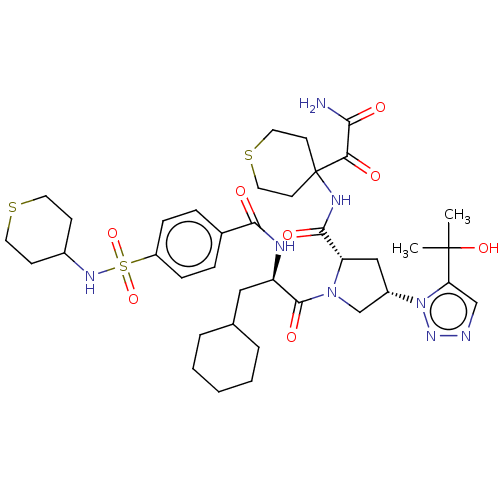 (US11377439, Example 113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560299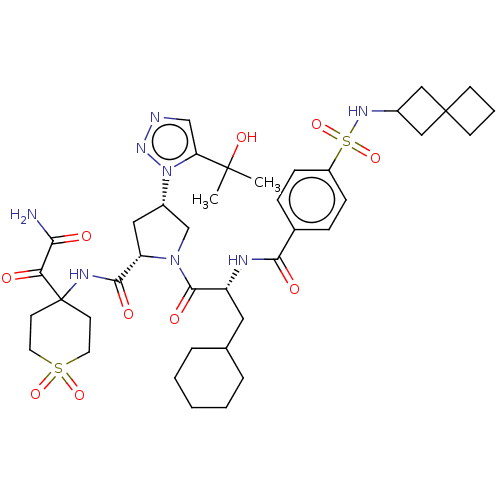 (US11377439, Example 136) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50240279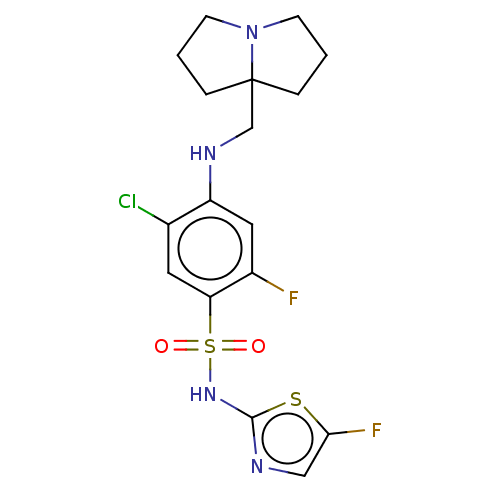 (CHEMBL4080553) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry Merck& Co. Curated by ChEMBL | Assay Description Inhibition of 50% inactivated human Nav1.7alpha expressed in HEK293 cells at holding potential of -60 mV incubated for 5 mins measured at 10 secs int... | Bioorg Med Chem Lett 27: 2087-2093 (2017) Article DOI: 10.1016/j.bmcl.2017.03.085 BindingDB Entry DOI: 10.7270/Q29025XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560137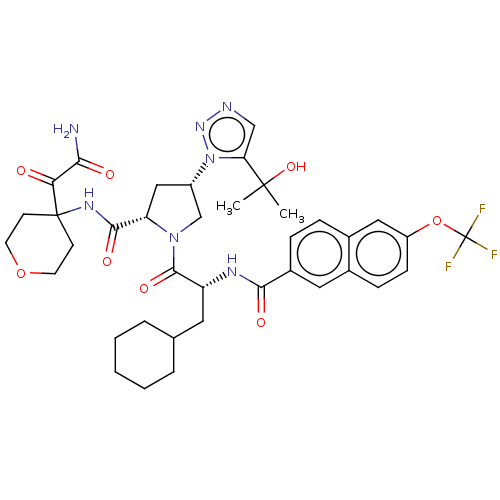 (US11377439, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560246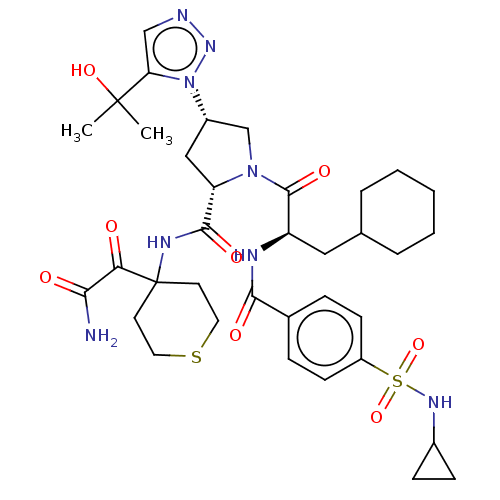 (US11377439, Example 83) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560281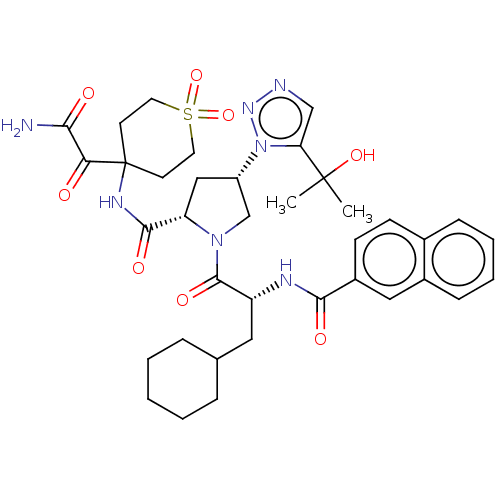 (US11377439, Example 118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560262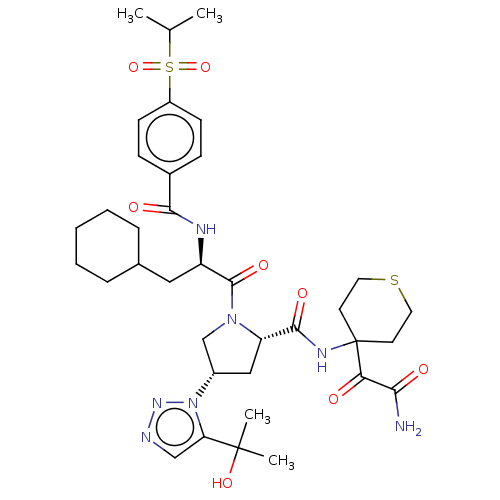 (US11377439, Example 99) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 735 total ) | Next | Last >> |