

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50440737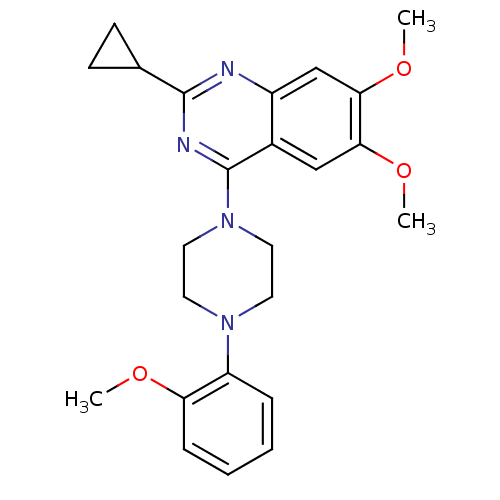 (CHEMBL2431120) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to sigma-1 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to 5-HT3 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to PBR receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of NTS1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of MOR (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DOR (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50444943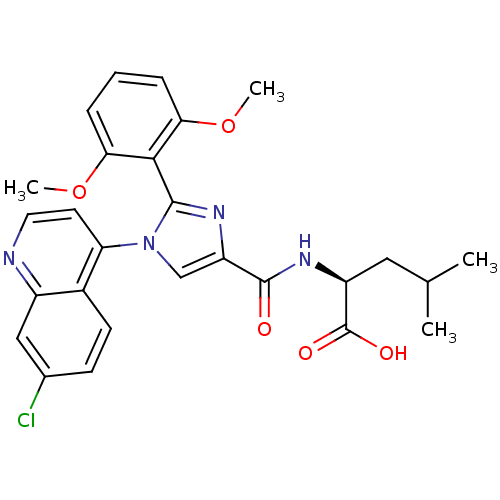 (CHEMBL3099773) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DAT (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444943 (CHEMBL3099773) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of NTS1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DAT (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50440738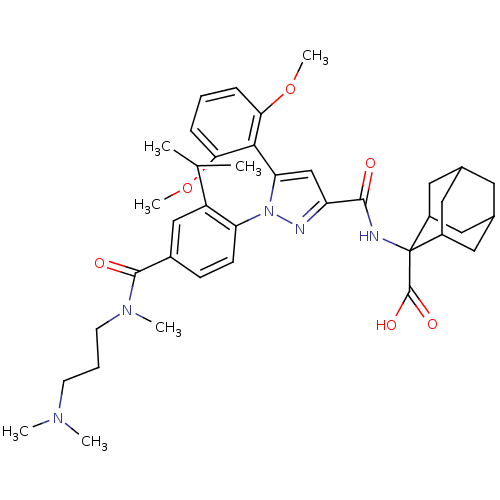 (CHEMBL2431105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-neurotensin from NTR1 (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50440738 (CHEMBL2431105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-neurotensin from NTR1 in HUVEC after 1 hr by gamma counting analysis | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50440738 (CHEMBL2431105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at NTR1 (unknown origin) expressed in human U2OS cells coexpressing beta-arrestin assessed as inhibition of ML314-induced effect ... | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50393964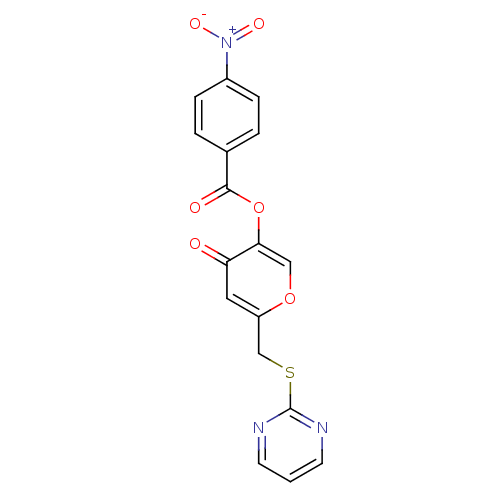 (CHEMBL2158347) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at APJ receptor assessed as inhibition of apelin13-induced cAMP accumulation | Bioorg Med Chem Lett 22: 6656-60 (2012) Article DOI: 10.1016/j.bmcl.2012.08.105 BindingDB Entry DOI: 10.7270/Q2H1333B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396489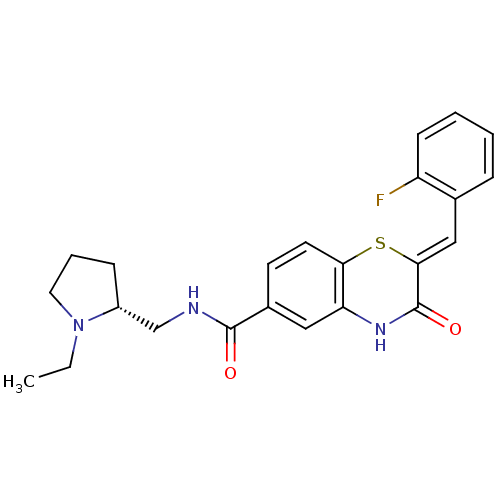 (CHEMBL2170943) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396486 (CHEMBL2171113) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396521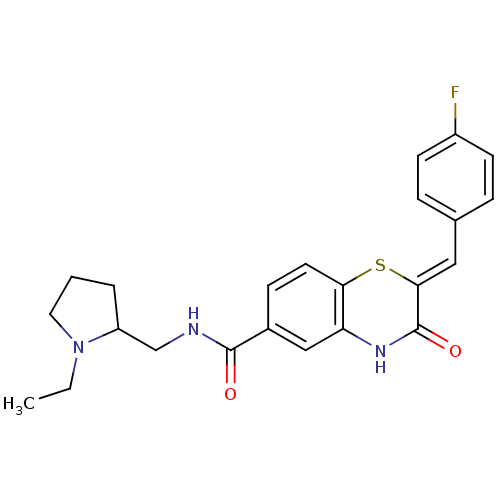 (CHEMBL2170936) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396519 (CHEMBL2170938) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396486 (CHEMBL2171113) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50393964 (CHEMBL2158347) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at APJ receptor expressed in CHOK1 cells assessed as inhibition of apelin13-induced beta-arrestin recruitment after 90 mins by lu... | Bioorg Med Chem Lett 22: 6656-60 (2012) Article DOI: 10.1016/j.bmcl.2012.08.105 BindingDB Entry DOI: 10.7270/Q2H1333B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM32307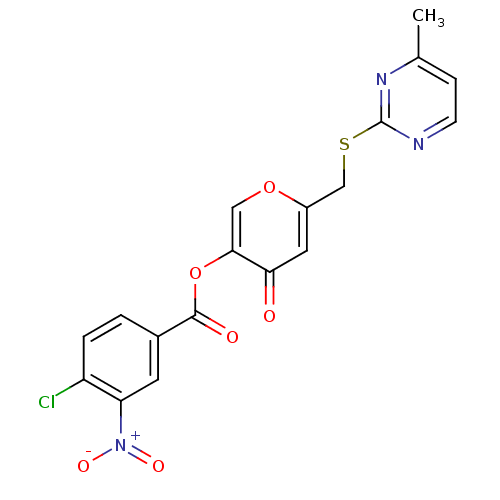 (4-chloro-3-nitro-benzoic acid [4-keto-6-[[(4-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at APJ receptor expressed in CHOK1 cells assessed as inhibition of apelin13-induced beta-arrestin recruitment after 90 mins by lu... | Bioorg Med Chem Lett 22: 6656-60 (2012) Article DOI: 10.1016/j.bmcl.2012.08.105 BindingDB Entry DOI: 10.7270/Q2H1333B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396489 (CHEMBL2170943) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396521 (CHEMBL2170936) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396522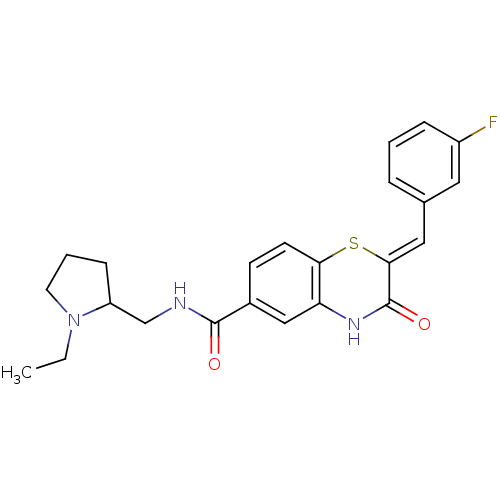 (CHEMBL2170935) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396517 (CHEMBL2170940) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396488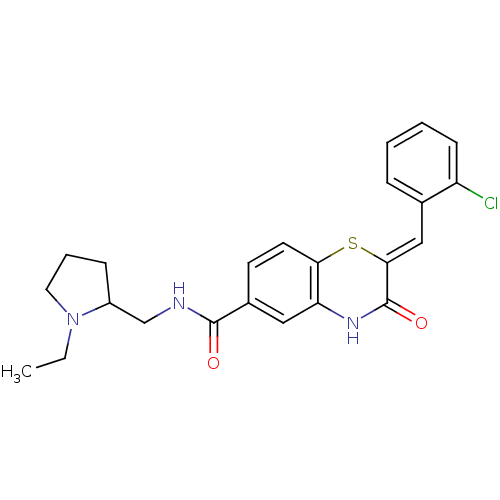 (CHEMBL2170929) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396519 (CHEMBL2170938) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396517 (CHEMBL2170940) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396515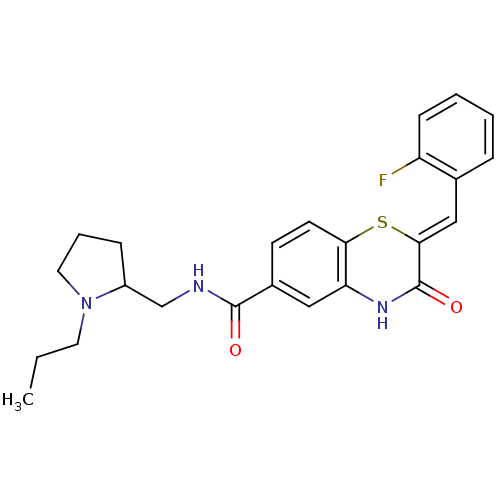 (CHEMBL2170945) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50393896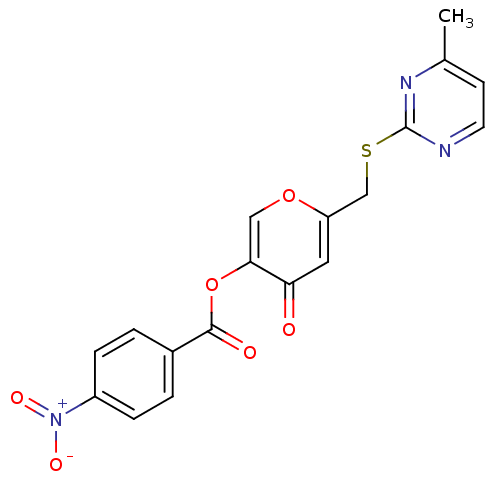 (CHEMBL2158275) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at APJ receptor expressed in CHOK1 cells assessed as inhibition of apelin13-induced beta-arrestin recruitment after 90 mins by lu... | Bioorg Med Chem Lett 22: 6656-60 (2012) Article DOI: 10.1016/j.bmcl.2012.08.105 BindingDB Entry DOI: 10.7270/Q2H1333B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50393932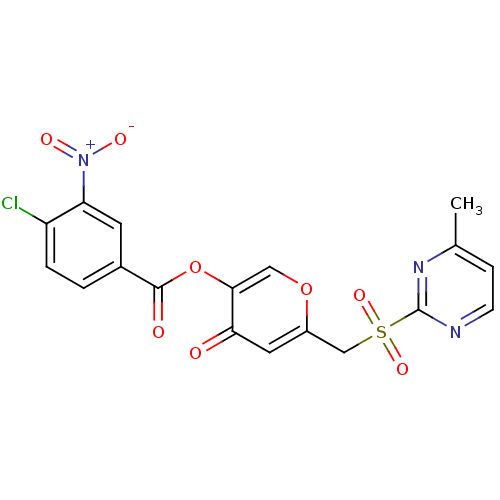 (CHEMBL2158342) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at APJ receptor expressed in CHOK1 cells assessed as inhibition of apelin13-induced beta-arrestin recruitment after 90 mins by lu... | Bioorg Med Chem Lett 22: 6656-60 (2012) Article DOI: 10.1016/j.bmcl.2012.08.105 BindingDB Entry DOI: 10.7270/Q2H1333B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396488 (CHEMBL2170929) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396520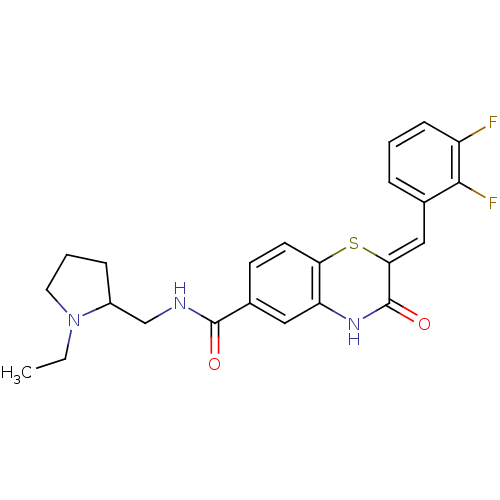 (CHEMBL2170937) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396515 (CHEMBL2170945) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396522 (CHEMBL2170935) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396518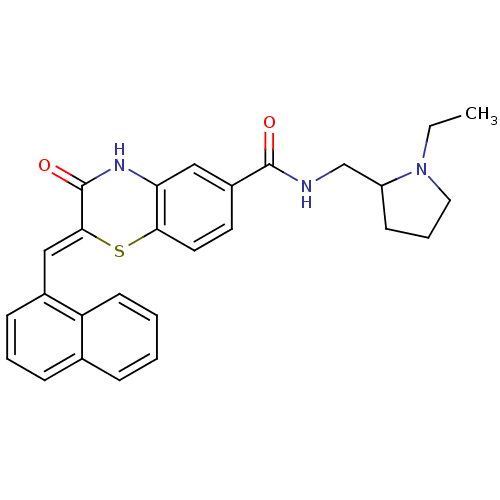 (CHEMBL2170939) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396518 (CHEMBL2170939) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396487 (CHEMBL2170930) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50393948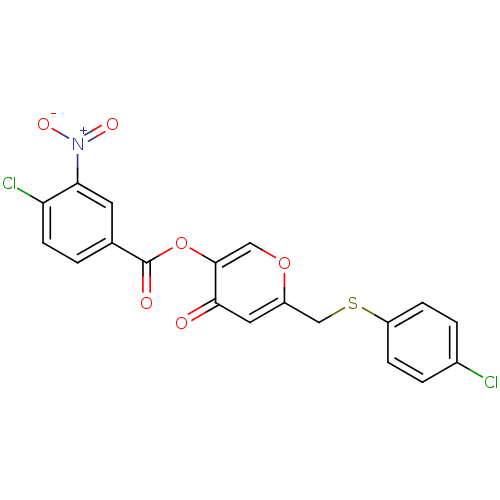 (CHEMBL2158327) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at APJ receptor expressed in CHOK1 cells assessed as inhibition of apelin13-induced beta-arrestin recruitment after 90 mins by lu... | Bioorg Med Chem Lett 22: 6656-60 (2012) Article DOI: 10.1016/j.bmcl.2012.08.105 BindingDB Entry DOI: 10.7270/Q2H1333B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50393899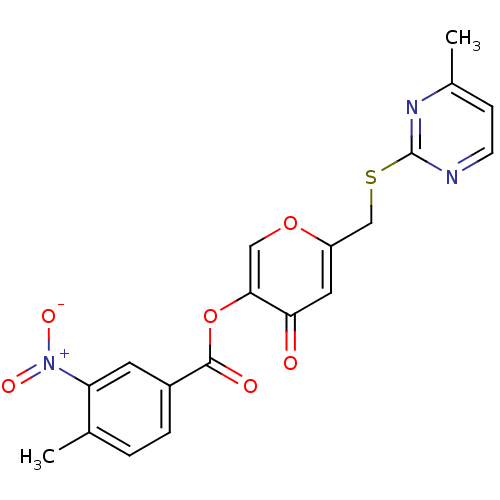 (CHEMBL2158272) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at APJ receptor expressed in CHOK1 cells assessed as inhibition of apelin13-induced beta-arrestin recruitment after 90 mins by lu... | Bioorg Med Chem Lett 22: 6656-60 (2012) Article DOI: 10.1016/j.bmcl.2012.08.105 BindingDB Entry DOI: 10.7270/Q2H1333B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396516 (CHEMBL2170944) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396508 (CHEMBL2170923) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396508 (CHEMBL2170923) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396513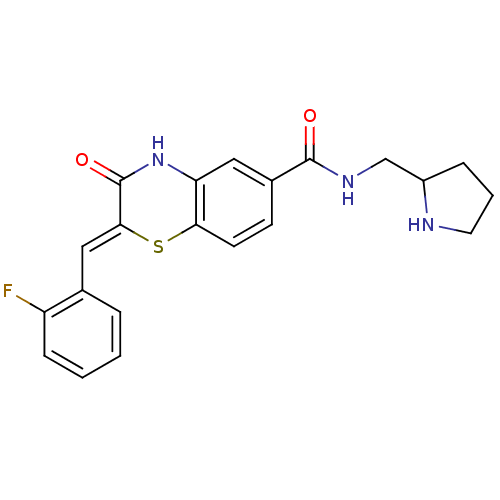 (CHEMBL2171111) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396524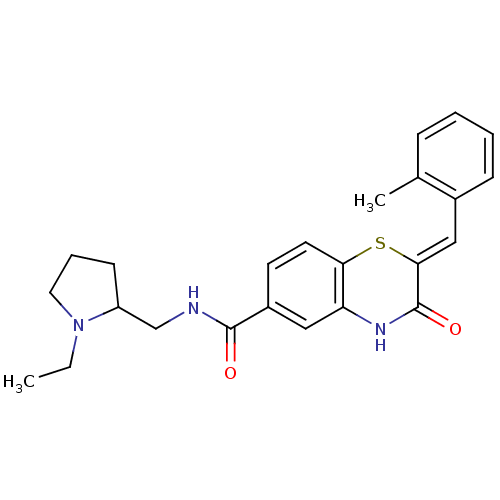 (CHEMBL2170933) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396520 (CHEMBL2170937) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396513 (CHEMBL2171111) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396524 (CHEMBL2170933) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50393925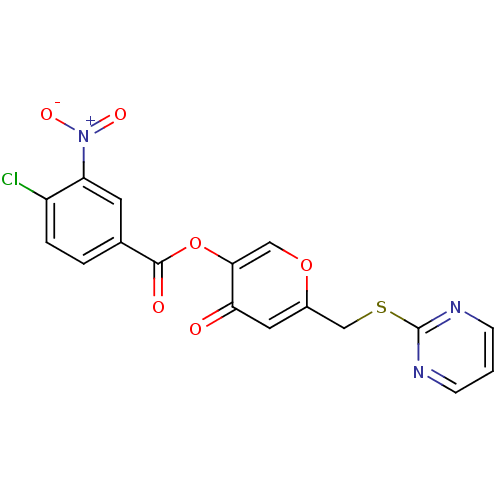 (CHEMBL2158344) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at APJ receptor expressed in CHOK1 cells assessed as inhibition of apelin13-induced beta-arrestin recruitment after 90 mins by lu... | Bioorg Med Chem Lett 22: 6656-60 (2012) Article DOI: 10.1016/j.bmcl.2012.08.105 BindingDB Entry DOI: 10.7270/Q2H1333B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50393921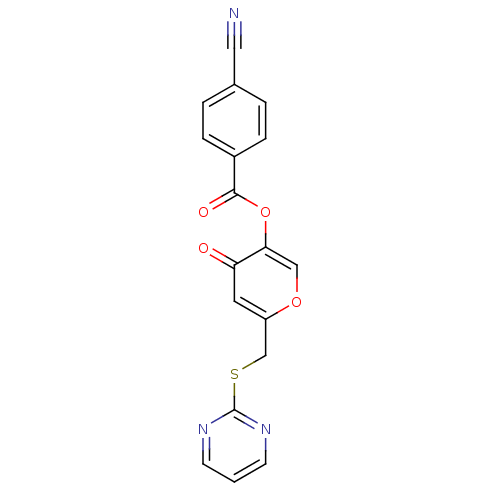 (CHEMBL2158348) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at APJ receptor expressed in CHOK1 cells assessed as inhibition of apelin13-induced beta-arrestin recruitment after 90 mins by lu... | Bioorg Med Chem Lett 22: 6656-60 (2012) Article DOI: 10.1016/j.bmcl.2012.08.105 BindingDB Entry DOI: 10.7270/Q2H1333B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 422 total ) | Next | Last >> |