

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Displacement of fluorescent estrogen ES2 from human recombinant ERbeta by fluorescence polarization assay | J Nat Prod 72: 1603-7 (2009) Article DOI: 10.1021/np900271m BindingDB Entry DOI: 10.7270/Q2D21XRF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Displacement of fluorescent estrogen ES2 from human recombinant ERalpha by fluorescence polarization assay | J Nat Prod 72: 1603-7 (2009) Article DOI: 10.1021/np900271m BindingDB Entry DOI: 10.7270/Q2D21XRF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50250229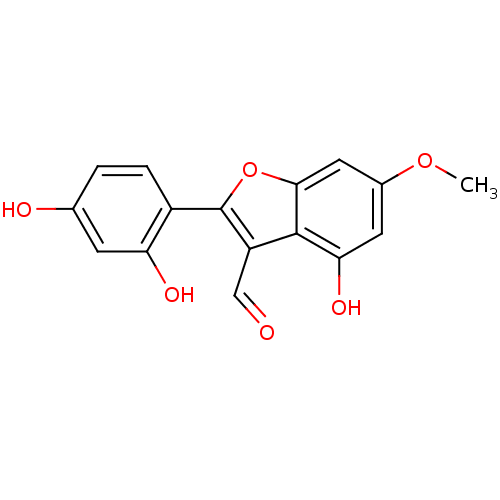 (2-(2,4-dihydroxyphenyl)-3-formyl-4-hydroxy-6-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from estrogen receptor (unknown origin) | J Nat Prod 63: 1672-4 (2001) BindingDB Entry DOI: 10.7270/Q2SB45HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50250228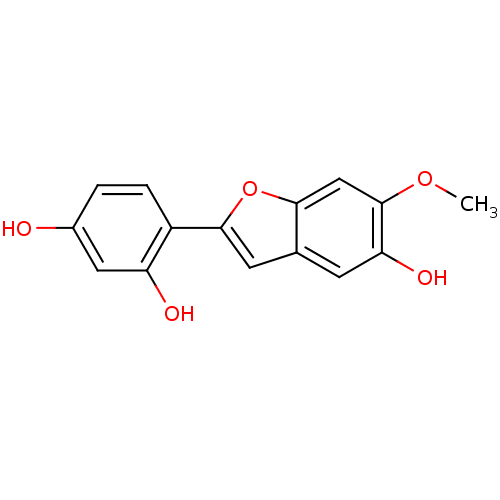 (2-(2,4-dihydroxyphenyl)-5-hydroxy-6-methoxy-benzof...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from estrogen receptor (unknown origin) | J Nat Prod 63: 1672-4 (2001) BindingDB Entry DOI: 10.7270/Q2SB45HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase using arachidonic acid as substrate preincubated for 10 mins before substrate addition measured after ... | J Nat Prod 77: 441-5 (2014) Article DOI: 10.1021/np401010x BindingDB Entry DOI: 10.7270/Q28K7BKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50357364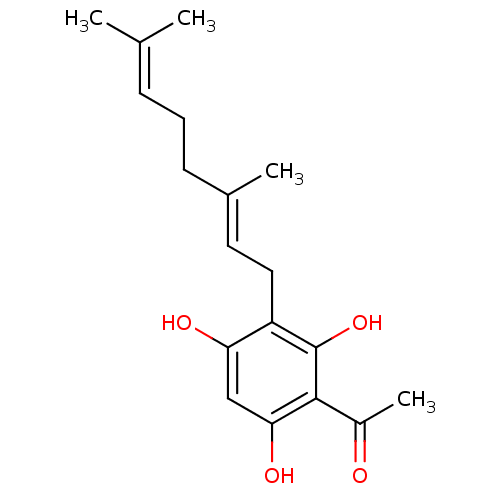 (CHEMBL1242095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267227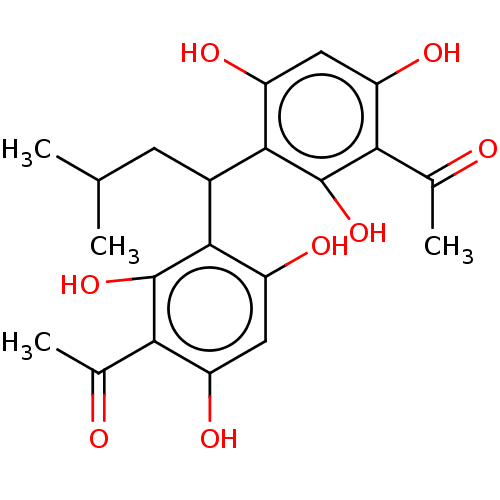 (CHEMBL4087914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267243 (CHEMBL1984096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267248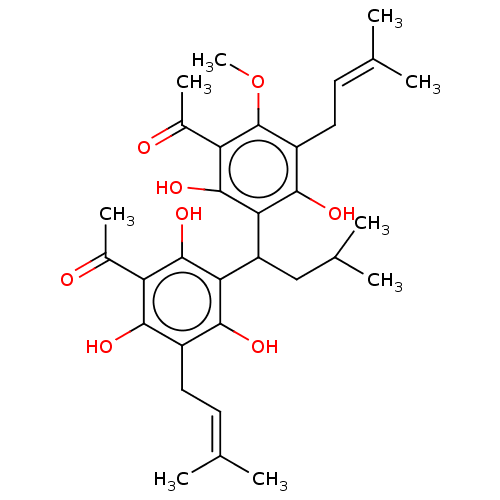 (CHEBI:2440 | CHEMBL488313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267233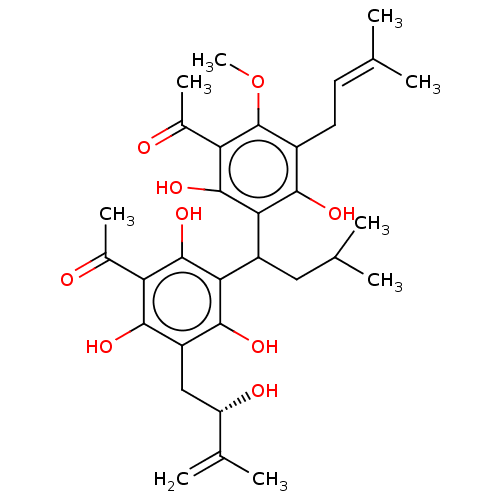 (CHEMBL4075212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267246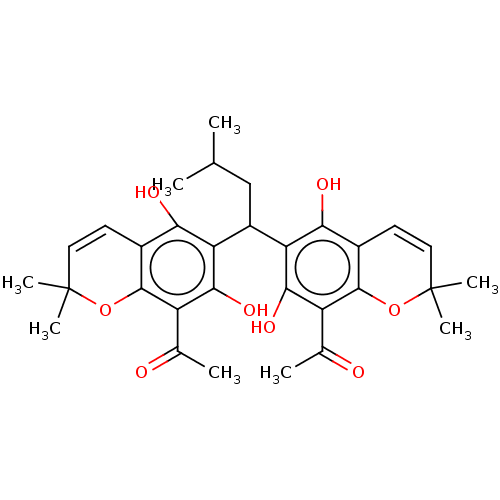 (CHEMBL4069303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267234 (Acrofolione A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50357364 (CHEMBL1242095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267227 (CHEMBL4087914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267244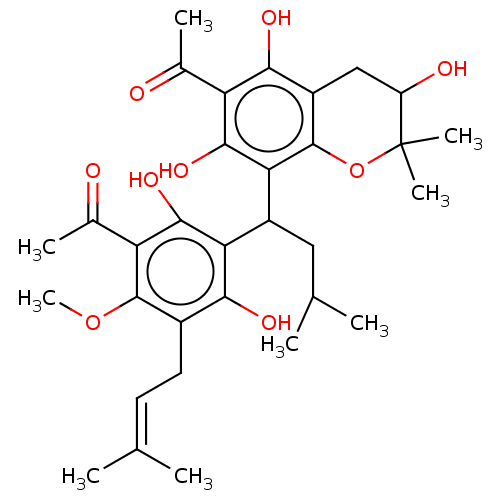 (CHEMBL4077368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267244 (CHEMBL4077368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50448447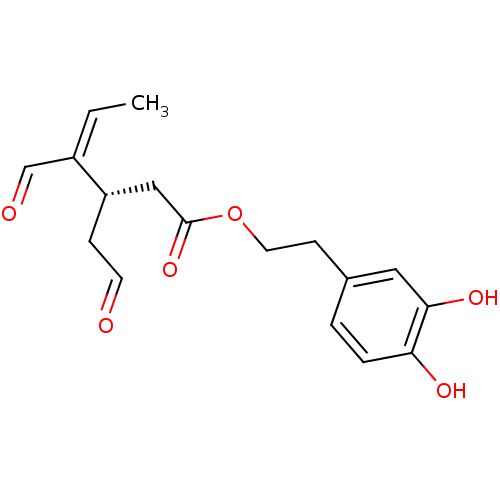 (CHEMBL3125444) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase using arachidonic acid as substrate preincubated for 10 mins before substrate addition measured after ... | J Nat Prod 77: 441-5 (2014) Article DOI: 10.1021/np401010x BindingDB Entry DOI: 10.7270/Q28K7BKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267248 (CHEBI:2440 | CHEMBL488313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267246 (CHEMBL4069303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267233 (CHEMBL4075212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267245 (CHEMBL4078358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267248 (CHEBI:2440 | CHEMBL488313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50357364 (CHEMBL1242095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267241 (CHEMBL4080027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267247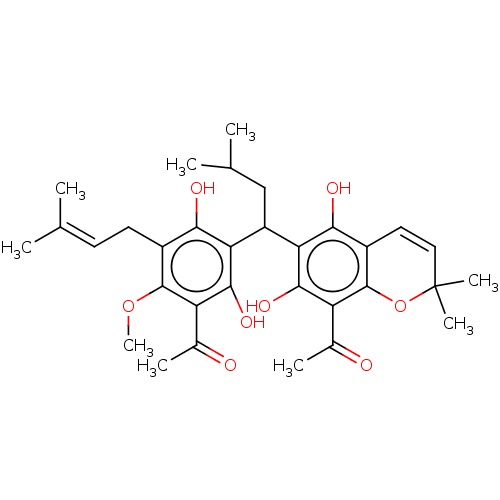 (CHEMBL4101532) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267243 (CHEMBL1984096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267244 (CHEMBL4077368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267242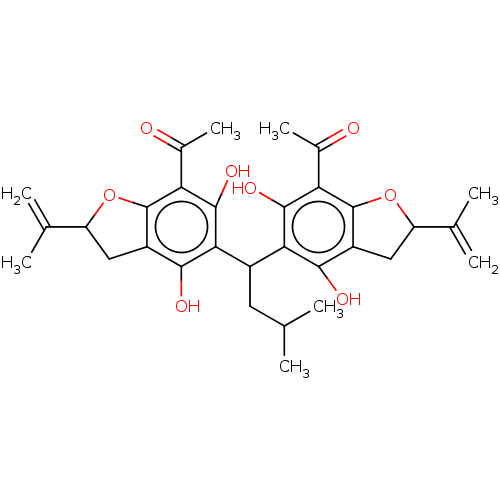 (CHEMBL4096534) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267245 (CHEMBL4078358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267234 (Acrofolione A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267241 (CHEMBL4080027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267247 (CHEMBL4101532) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267227 (CHEMBL4087914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267233 (CHEMBL4075212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267234 (Acrofolione A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267245 (CHEMBL4078358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267246 (CHEMBL4069303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267247 (CHEMBL4101532) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267241 (CHEMBL4080027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267240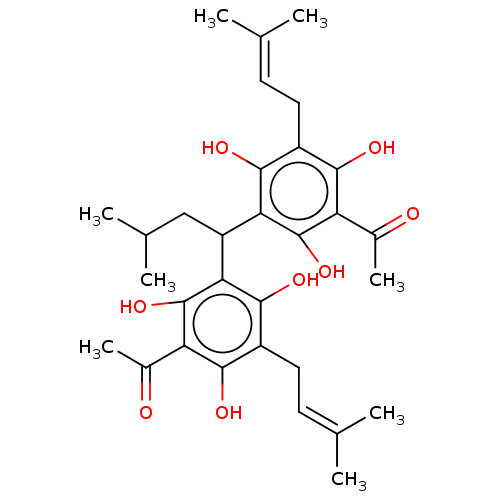 (CHEMBL4104264) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267242 (CHEMBL4096534) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267243 (CHEMBL1984096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267240 (CHEMBL4104264) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267240 (CHEMBL4104264) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50267232 (CHEMBL1241050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human A549 cell microsomal membrane-derived mPGES-1 assessed as reduction in conversion of PGH2 to PGE2 preincubated for 15 mins follow... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50267242 (CHEMBL4096534) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli MV1190 using arachidonic acid as substrate preincubated for 10 mins followed by su... | J Nat Prod 80: 699-706 (2017) Article DOI: 10.1021/acs.jnatprod.6b01008 BindingDB Entry DOI: 10.7270/Q2J38W29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50448446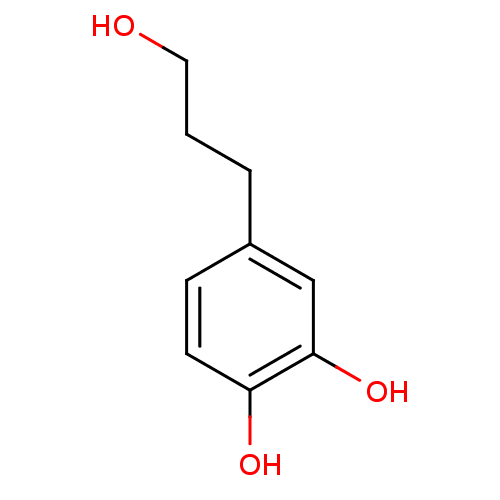 (Hydroxytyrosol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase using arachidonic acid as substrate preincubated for 10 mins before substrate addition measured after ... | J Nat Prod 77: 441-5 (2014) Article DOI: 10.1021/np401010x BindingDB Entry DOI: 10.7270/Q28K7BKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||