

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50041289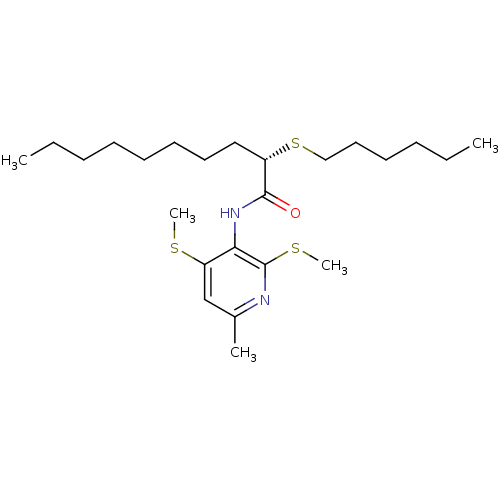 ((S)-2-Hexylsulfanyl-decanoic acid (6-methyl-2,4-bi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro potency was determined using Acyl coenzyme A:cholesterol acyltransferase in liver microsomes from rats | J Med Chem 37: 1252-5 (1994) BindingDB Entry DOI: 10.7270/Q25X280J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50097898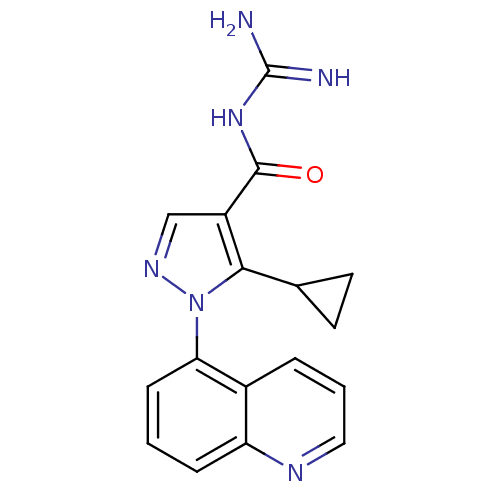 (CHEMBL355862 | N-(5-Cyclopropyl-1-quinolin-5-yl-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit NHE-1 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50041290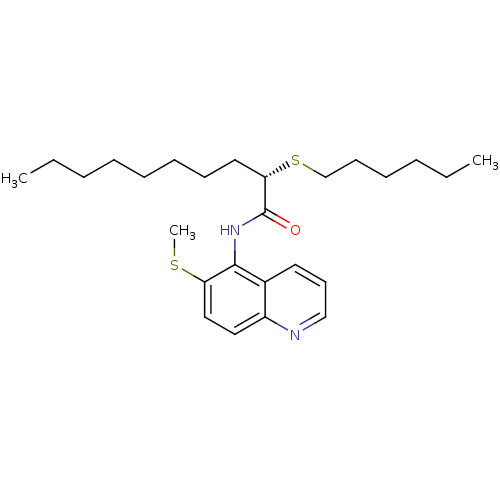 ((S)-2-Hexylsulfanyl-decanoic acid (6-methylsulfany...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro potency was determined using Acyl coenzyme A:cholesterol acyltransferase in liver microsomes from rats | J Med Chem 37: 1252-5 (1994) BindingDB Entry DOI: 10.7270/Q25X280J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50058715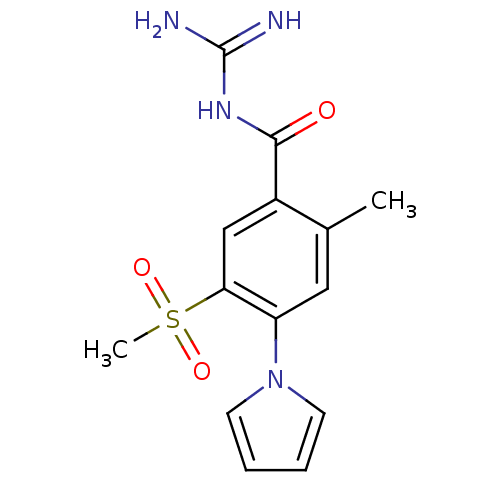 (CHEMBL64360 | EMD-96785 | ENIPORIDE | N-(5-Methane...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit NHE-1 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50453642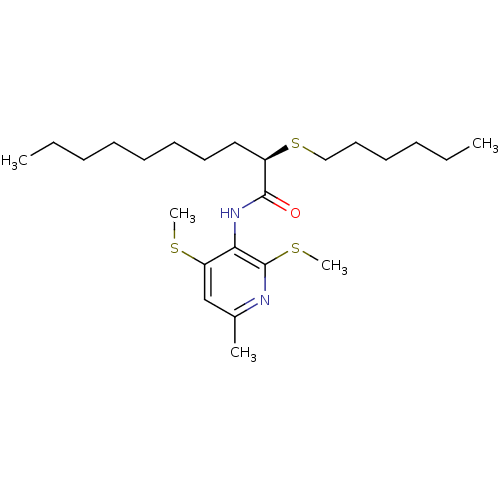 (CHEMBL2093028) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro potency was determined using Acyl coenzyme A:cholesterol acyltransferase in liver microsomes from rats | J Med Chem 37: 1252-5 (1994) BindingDB Entry DOI: 10.7270/Q25X280J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50097896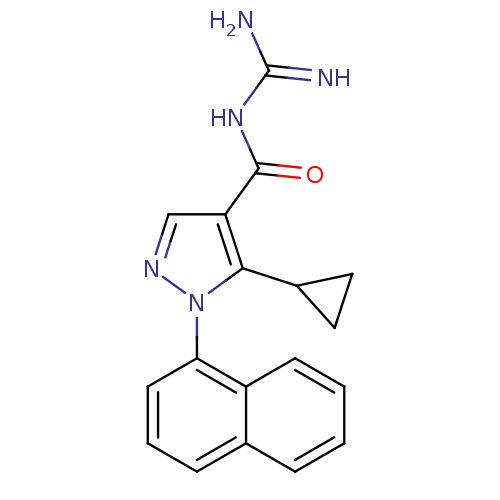 (CHEMBL354137 | N-(5-Cyclopropyl-1-naphthalen-1-yl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit NHE-1 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50097895 (CHEMBL355071 | N-(5-Cyclopropyl-1-phenyl-1H-pyrazo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit NHE-1 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50058759 (CHEMBL436559 | CHEMBL462831 | Cariporide | N-(4-Is...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit NHE-1 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50453641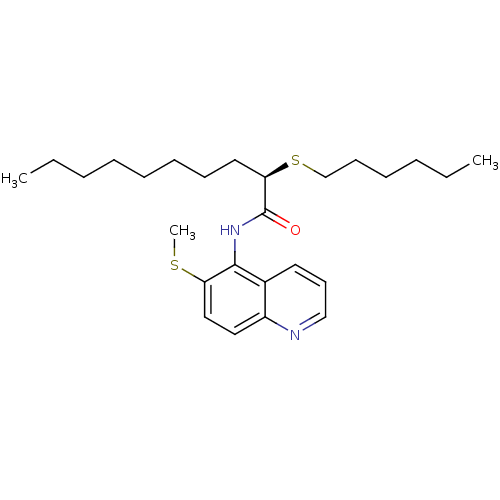 (CHEMBL2093029) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro potency was determined using Acyl coenzyme A:cholesterol acyltransferase in liver microsomes from rats | J Med Chem 37: 1252-5 (1994) BindingDB Entry DOI: 10.7270/Q25X280J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50097897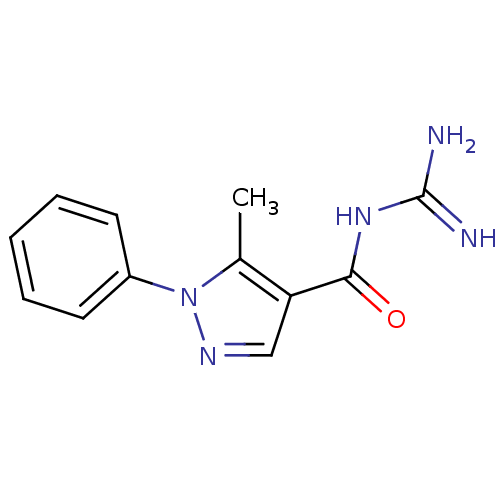 (CHEMBL352933 | N-(5-Methyl-1-phenyl-1H-pyrazole-4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit NHE-1 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50097899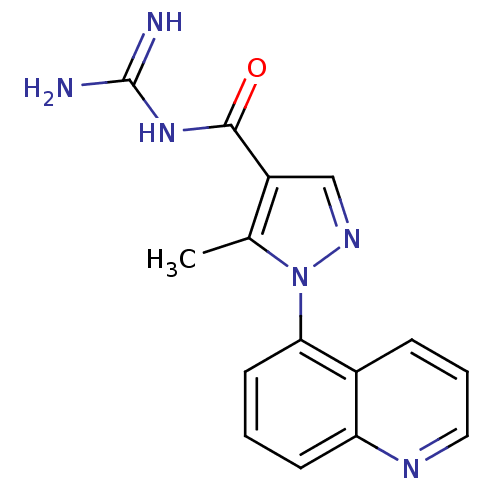 (CHEMBL167495 | N-(5-Methyl-1-quinolin-5-yl-1H-pyra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit NHE-1 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 2 (Homo sapiens (Human)) | BDBM50097897 (CHEMBL352933 | N-(5-Methyl-1-phenyl-1H-pyrazole-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration of compound required to inhibit NHE-2 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 2 (Homo sapiens (Human)) | BDBM50058715 (CHEMBL64360 | EMD-96785 | ENIPORIDE | N-(5-Methane...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration of compound required to inhibit NHE-2 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 2 (Homo sapiens (Human)) | BDBM50097898 (CHEMBL355862 | N-(5-Cyclopropyl-1-quinolin-5-yl-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration of compound required to inhibit NHE-2 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 2 (Homo sapiens (Human)) | BDBM50097895 (CHEMBL355071 | N-(5-Cyclopropyl-1-phenyl-1H-pyrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration of compound required to inhibit NHE-2 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 2 (Homo sapiens (Human)) | BDBM50097896 (CHEMBL354137 | N-(5-Cyclopropyl-1-naphthalen-1-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration of compound required to inhibit NHE-2 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 2 (Homo sapiens (Human)) | BDBM50058759 (CHEMBL436559 | CHEMBL462831 | Cariporide | N-(4-Is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration of compound required to inhibit NHE-2 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 2 (Homo sapiens (Human)) | BDBM50097899 (CHEMBL167495 | N-(5-Methyl-1-quinolin-5-yl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration of compound required to inhibit NHE-2 mediated intracellular maximal pH recovery by 50% | Bioorg Med Chem Lett 11: 803-7 (2001) BindingDB Entry DOI: 10.7270/Q2TM79CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||