 Found 357 hits with Last Name = 'haupenthal' and Initial = 'j'
Found 357 hits with Last Name = 'haupenthal' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50589973
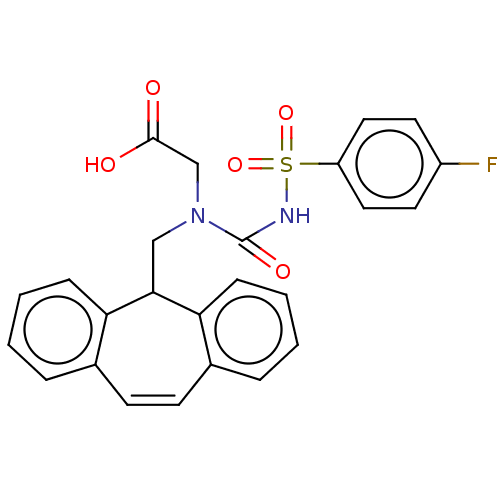
(CHEMBL5184569)Show SMILES OC(=O)CN(CC1c2ccccc2C=Cc2ccccc12)C(=O)NS(=O)(=O)c1ccc(F)cc1 |c:14| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50589973

(CHEMBL5184569)Show SMILES OC(=O)CN(CC1c2ccccc2C=Cc2ccccc12)C(=O)NS(=O)(=O)c1ccc(F)cc1 |c:14| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50542712
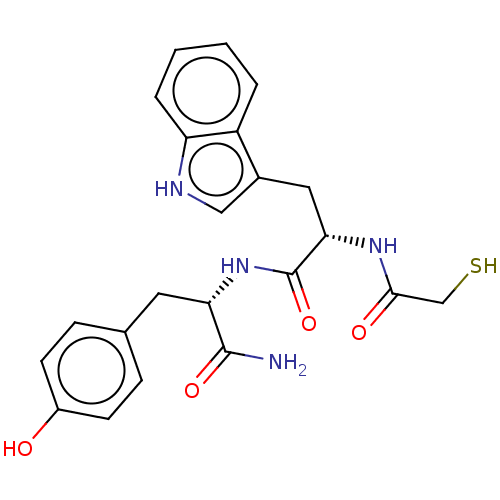
(CHEMBL4647152)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CS |r| Show InChI InChI=1S/C22H24N4O4S/c23-21(29)18(9-13-5-7-15(27)8-6-13)26-22(30)19(25-20(28)12-31)10-14-11-24-17-4-2-1-3-16(14)17/h1-8,11,18-19,24,27,31H,9-10,12H2,(H2,23,29)(H,25,28)(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) - Helmholtz Centre for Infection Research (HZI)
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LasB using aminobenzoyl-Ala-Gly-Leu-Ala-p-nitro-benzyl-amide as fluorogenic substrate by fluorimetric assay |
J Med Chem 63: 8359-8368 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00584
BindingDB Entry DOI: 10.7270/Q20005N6 |
More data for this
Ligand-Target Pair | |
Collagenase ColQ1
(Bacillus cereus (strain Q1)) | BDBM50589971

(CHEMBL5187038)Show SMILES CC(C)CC(C(=O)NO)C(=O)NCCn1cc(COc2ccccc2NC(C)=O)nn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM11859
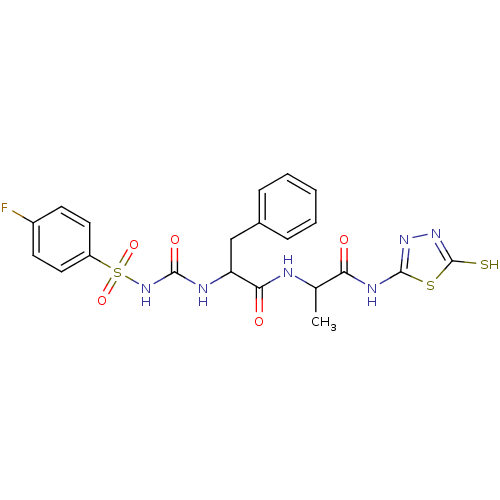
(2-({[(4-fluorobenzene)sulfonyl]carbamoyl}amino)-3-...)Show SMILES CC(NC(=O)C(Cc1ccccc1)NC(=O)NS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C21H21FN6O5S3/c1-12(17(29)25-20-26-27-21(34)35-20)23-18(30)16(11-13-5-3-2-4-6-13)24-19(31)28-36(32,33)15-9-7-14(22)8-10-15/h2-10,12,16H,11H2,1H3,(H,23,30)(H,27,34)(H2,24,28,31)(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Collagenase ColH
(Hathewaya histolytica) | BDBM11859

(2-({[(4-fluorobenzene)sulfonyl]carbamoyl}amino)-3-...)Show SMILES CC(NC(=O)C(Cc1ccccc1)NC(=O)NS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C21H21FN6O5S3/c1-12(17(29)25-20-26-27-21(34)35-20)23-18(30)16(11-13-5-3-2-4-6-13)24-19(31)28-36(32,33)15-9-7-14(22)8-10-15/h2-10,12,16H,11H2,1H3,(H,23,30)(H,27,34)(H2,24,28,31)(H,25,26,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Collagenase ColQ1
(Bacillus cereus (strain Q1)) | BDBM50589972
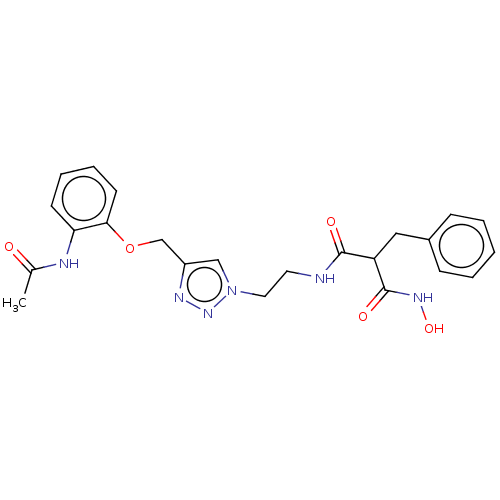
(CHEMBL5191074)Show SMILES CC(=O)Nc1ccccc1OCc1cn(CCNC(=O)C(Cc2ccccc2)C(=O)NO)nn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Collagenase ColH
(Hathewaya histolytica) | BDBM50589972

(CHEMBL5191074)Show SMILES CC(=O)Nc1ccccc1OCc1cn(CCNC(=O)C(Cc2ccccc2)C(=O)NO)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11859

(2-({[(4-fluorobenzene)sulfonyl]carbamoyl}amino)-3-...)Show SMILES CC(NC(=O)C(Cc1ccccc1)NC(=O)NS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C21H21FN6O5S3/c1-12(17(29)25-20-26-27-21(34)35-20)23-18(30)16(11-13-5-3-2-4-6-13)24-19(31)28-36(32,33)15-9-7-14(22)8-10-15/h2-10,12,16H,11H2,1H3,(H,23,30)(H,27,34)(H2,24,28,31)(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Collagenase ColA
(Bacillus cereus (strain ATCC 14579 / DSM 31 / CCUG...) | BDBM50589971

(CHEMBL5187038)Show SMILES CC(C)CC(C(=O)NO)C(=O)NCCn1cc(COc2ccccc2NC(C)=O)nn1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Collagenase ColA
(Bacillus cereus (strain ATCC 14579 / DSM 31 / CCUG...) | BDBM50589972

(CHEMBL5191074)Show SMILES CC(=O)Nc1ccccc1OCc1cn(CCNC(=O)C(Cc2ccccc2)C(=O)NO)nn1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Collagenase ColH
(Hathewaya histolytica) | BDBM50589971

(CHEMBL5187038)Show SMILES CC(C)CC(C(=O)NO)C(=O)NCCn1cc(COc2ccccc2NC(C)=O)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Collagenase ColG
(Clostridium histolyticum) | BDBM50589972

(CHEMBL5191074)Show SMILES CC(=O)Nc1ccccc1OCc1cn(CCNC(=O)C(Cc2ccccc2)C(=O)NO)nn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Collagenase ColG
(Clostridium histolyticum) | BDBM50589971

(CHEMBL5187038)Show SMILES CC(C)CC(C(=O)NO)C(=O)NCCn1cc(COc2ccccc2NC(C)=O)nn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50125935
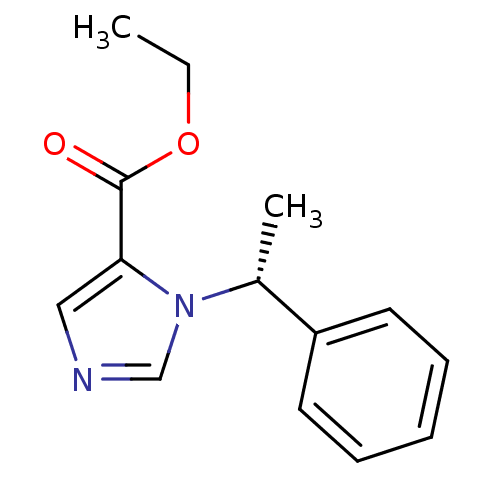
((R)-1-(1-phenylethyl)-1H-imidazole-5-carboxylic ac...)Show InChI InChI=1S/C14H16N2O2/c1-3-18-14(17)13-9-15-10-16(13)11(2)12-7-5-4-6-8-12/h4-11H,3H2,1-2H3/t11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50125935

((R)-1-(1-phenylethyl)-1H-imidazole-5-carboxylic ac...)Show InChI InChI=1S/C14H16N2O2/c1-3-18-14(17)13-9-15-10-16(13)11(2)12-7-5-4-6-8-12/h4-11H,3H2,1-2H3/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Collagenase ColH
(Hathewaya histolytica) | BDBM50589974
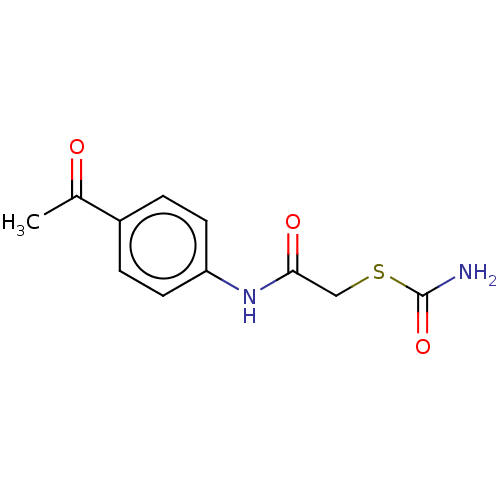
(CHEMBL5186191) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50028166
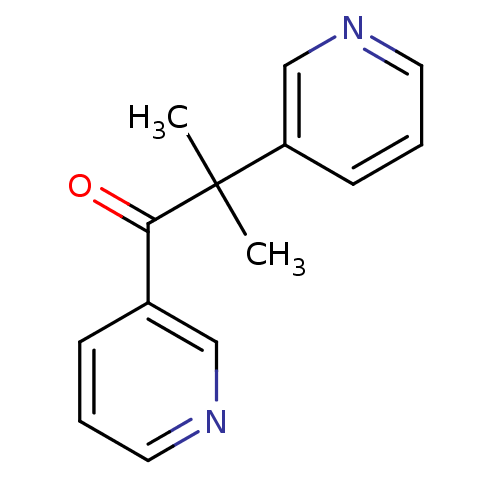
(CHEMBL934 | METYRAPONE | US9138393, Metyrapone | U...)Show InChI InChI=1S/C14H14N2O/c1-14(2,12-6-4-8-16-10-12)13(17)11-5-3-7-15-9-11/h3-10H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50141090
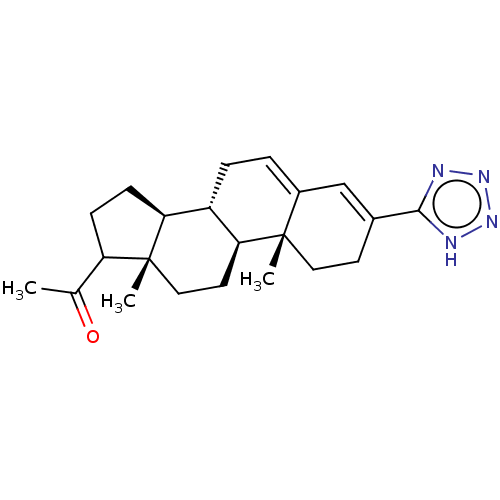
(CHEMBL3753791)Show SMILES [H][C@@]12CCC(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C=C(CC[C@]12C)c1nnn[nH]1 |r,c:21,t:19| Show InChI InChI=1S/C22H30N4O/c1-13(27)17-6-7-18-16-5-4-15-12-14(20-23-25-26-24-20)8-10-21(15,2)19(16)9-11-22(17,18)3/h4,12,16-19H,5-11H2,1-3H3,(H,23,24,25,26)/t16-,17?,18-,19-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human 5alpha-2 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by... |
Bioorg Med Chem 24: 779-88 (2016)
Article DOI: 10.1016/j.bmc.2015.12.048
BindingDB Entry DOI: 10.7270/Q21G0P3C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378948

(CHEMBL2011431 | US9394290, 36)Show InChI InChI=1S/C13H11N3S/c1-3-11(9-16-7-6-14-10-16)13(15-5-1)12-4-2-8-17-12/h1-8,10H,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Collagenase ColH
(Hathewaya histolytica) | BDBM50542711
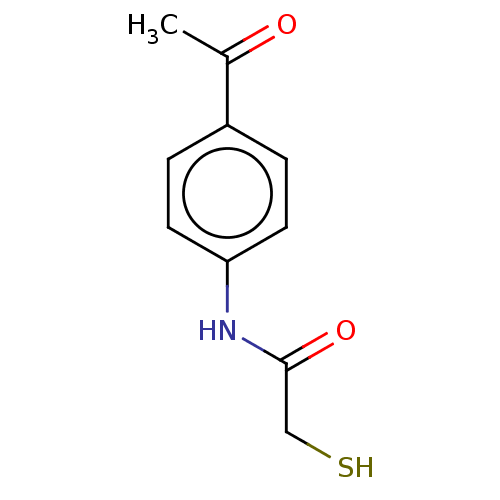
(CHEMBL4640494)Show InChI InChI=1S/C10H11NO2S/c1-7(12)8-2-4-9(5-3-8)11-10(13)6-14/h2-5,14H,6H2,1H3,(H,11,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase ColH
(Hathewaya histolytica) | BDBM50542711

(CHEMBL4640494)Show InChI InChI=1S/C10H11NO2S/c1-7(12)8-2-4-9(5-3-8)11-10(13)6-14/h2-5,14H,6H2,1H3,(H,11,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) - Helmholtz Centre for Infection Research (HZI)
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium histolyticum ColH peptidase domain expressed in Escherichia coli BL21(DE3) cells using Mca-Ala-Gly-Pro-Pro-Gly-Pro-Dpa-Gly-... |
J Med Chem 63: 8359-8368 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00584
BindingDB Entry DOI: 10.7270/Q20005N6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378956
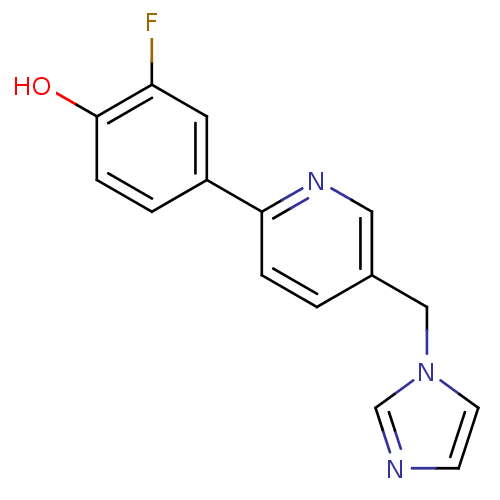
(CHEMBL2011246 | US9394290, 11)Show InChI InChI=1S/C15H12FN3O/c16-13-7-12(2-4-15(13)20)14-3-1-11(8-18-14)9-19-6-5-17-10-19/h1-8,10,20H,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Collagenase ColH
(Hathewaya histolytica) | BDBM50589973

(CHEMBL5184569)Show SMILES OC(=O)CN(CC1c2ccccc2C=Cc2ccccc12)C(=O)NS(=O)(=O)c1ccc(F)cc1 |c:14| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50396331
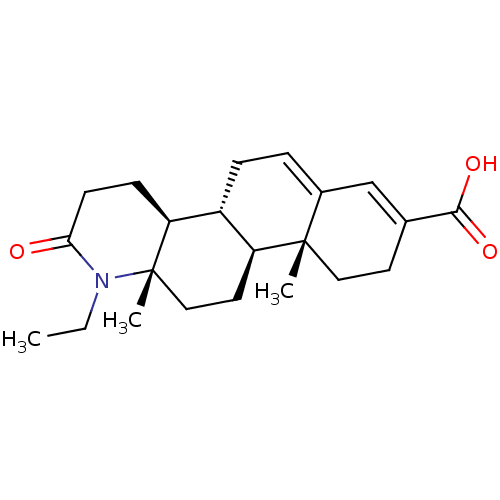
(CHEMBL2172644)Show SMILES CCN1C(=O)CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |r,c:12,t:10| Show InChI InChI=1S/C22H31NO3/c1-4-23-19(24)8-7-18-16-6-5-15-13-14(20(25)26)9-11-21(15,2)17(16)10-12-22(18,23)3/h5,13,16-18H,4,6-12H2,1-3H3,(H,25,26)/t16-,17+,18+,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of human type-2 5-alpha reductase expressed in HEK293 cells using [3H]-androstenedione as substrate after 30 mins by HPLC analysis |
Eur J Med Chem 54: 728-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.026
BindingDB Entry DOI: 10.7270/Q26T0NSX |
More data for this
Ligand-Target Pair | |
Collagenase ColH
(Hathewaya histolytica) | BDBM50361374
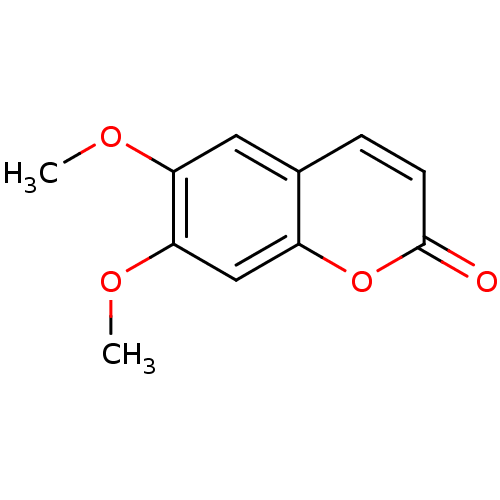
(SCOPARONE)Show InChI InChI=1S/C11H10O4/c1-13-9-5-7-3-4-11(12)15-8(7)6-10(9)14-2/h3-6H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
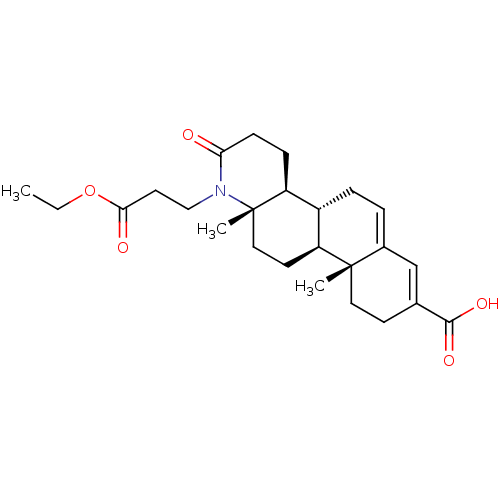
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50396329
(CHEMBL2172647)Show SMILES CCOC(=O)CCN1C(=O)CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |r,c:17,t:15| Show InChI InChI=1S/C25H35NO5/c1-4-31-22(28)11-14-26-21(27)8-7-20-18-6-5-17-15-16(23(29)30)9-12-24(17,2)19(18)10-13-25(20,26)3/h5,15,18-20H,4,6-14H2,1-3H3,(H,29,30)/t18-,19+,20+,24+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of human type-2 5-alpha reductase expressed in HEK293 cells using [3H]-androstenedione as substrate after 30 mins by HPLC analysis |
Eur J Med Chem 54: 728-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.026
BindingDB Entry DOI: 10.7270/Q26T0NSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378954

(CHEMBL2011437 | US9394290, 42)Show InChI InChI=1S/C17H13N3O2/c1-3-15(21-7-1)14-9-13(11-20-6-5-18-12-20)10-19-17(14)16-4-2-8-22-16/h1-10,12H,11H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 30.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of human type-2 5-alpha reductase expressed in HEK293 cells using [3H]-androstenedione as substrate after 30 mins by HPLC analysis |
Eur J Med Chem 54: 728-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.026
BindingDB Entry DOI: 10.7270/Q26T0NSX |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human 5alpha-2 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by... |
Bioorg Med Chem 24: 779-88 (2016)
Article DOI: 10.1016/j.bmc.2015.12.048
BindingDB Entry DOI: 10.7270/Q21G0P3C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378950
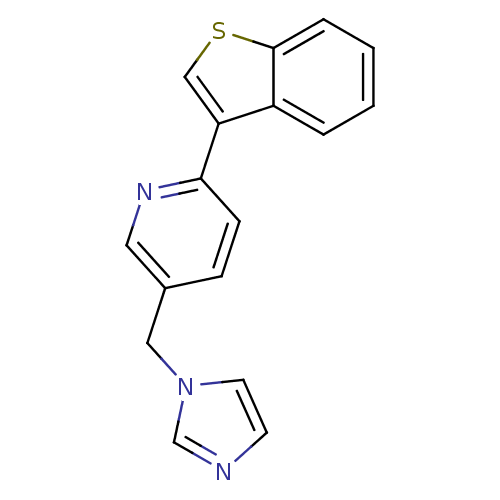
(CHEMBL2011433 | US9394290, 38)Show InChI InChI=1S/C17H13N3S/c1-2-4-17-14(3-1)15(11-21-17)16-6-5-13(9-19-16)10-20-8-7-18-12-20/h1-9,11-12H,10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378937
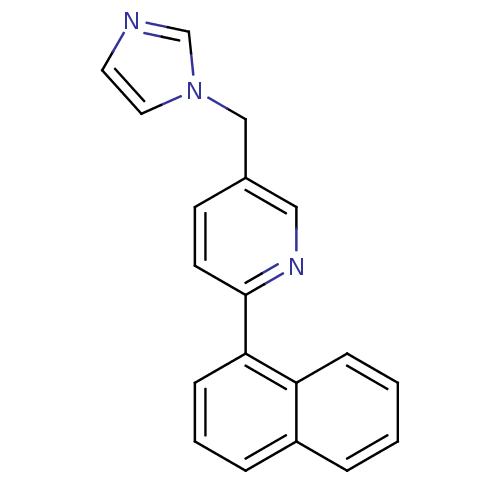
(CHEMBL2011253 | US9394290, 23)Show InChI InChI=1S/C19H15N3/c1-2-6-17-16(4-1)5-3-7-18(17)19-9-8-15(12-21-19)13-22-11-10-20-14-22/h1-12,14H,13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322793
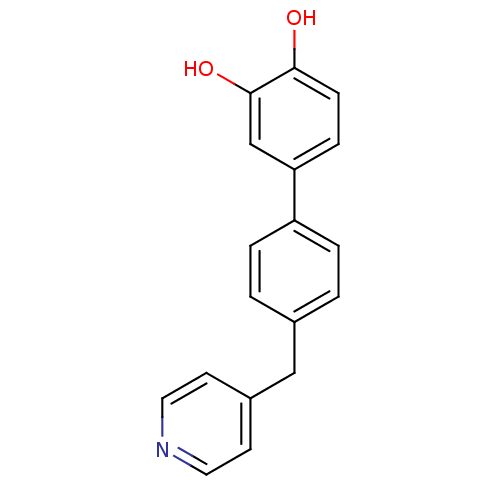
(4'-(Pyridin-4-ylmethyl)biphenyl-3,4-diol | CHEMBL1...)Show InChI InChI=1S/C18H15NO2/c20-17-6-5-16(12-18(17)21)15-3-1-13(2-4-15)11-14-7-9-19-10-8-14/h1-10,12,20-21H,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50396332
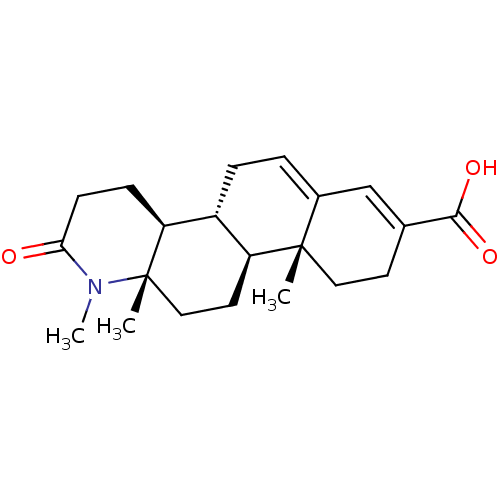
(CHEMBL2172643)Show SMILES CN1C(=O)CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |r,c:11,t:9| Show InChI InChI=1S/C21H29NO3/c1-20-10-8-13(19(24)25)12-14(20)4-5-15-16(20)9-11-21(2)17(15)6-7-18(23)22(21)3/h4,12,15-17H,5-11H2,1-3H3,(H,24,25)/t15-,16+,17+,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of human type-2 5-alpha reductase expressed in HEK293 cells using [3H]-androstenedione as substrate after 30 mins by HPLC analysis |
Eur J Med Chem 54: 728-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.026
BindingDB Entry DOI: 10.7270/Q26T0NSX |
More data for this
Ligand-Target Pair | |
Collagenase ColH
(Hathewaya histolytica) | BDBM50542698
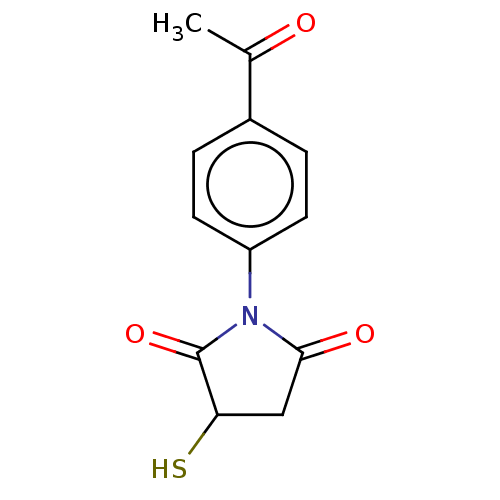
(CHEMBL4636059)Show InChI InChI=1S/C12H11NO3S/c1-7(14)8-2-4-9(5-3-8)13-11(15)6-10(17)12(13)16/h2-5,10,17H,6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) - Helmholtz Centre for Infection Research (HZI)
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium histolyticum ColH peptidase domain expressed in Escherichia coli BL21(DE3) cells using Mca-Ala-Gly-Pro-Pro-Gly-Pro-Dpa-Gly-... |
J Med Chem 63: 8359-8368 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00584
BindingDB Entry DOI: 10.7270/Q20005N6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378960
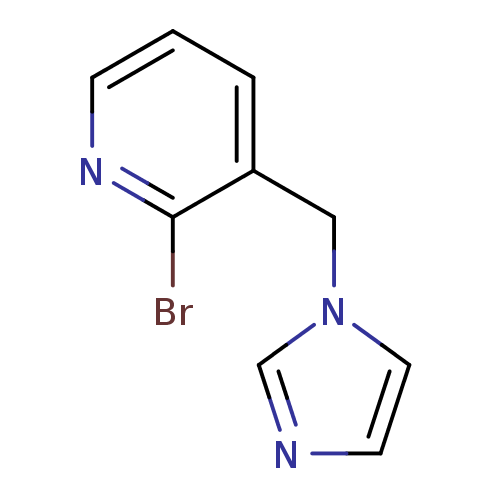
(CHEMBL2011242 | US9394290, 5)Show InChI InChI=1S/C9H8BrN3/c10-9-8(2-1-3-12-9)6-13-5-4-11-7-13/h1-5,7H,6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378946
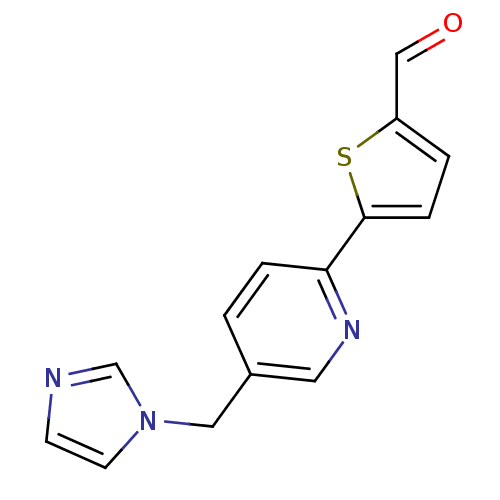
(CHEMBL2011264 | US9394290, 34)Show InChI InChI=1S/C14H11N3OS/c18-9-12-2-4-14(19-12)13-3-1-11(7-16-13)8-17-6-5-15-10-17/h1-7,9-10H,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50028166

(CHEMBL934 | METYRAPONE | US9138393, Metyrapone | U...)Show InChI InChI=1S/C14H14N2O/c1-14(2,12-6-4-8-16-10-12)13(17)11-5-3-7-15-9-11/h3-10H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378962

(CHEMBL2011244 | US9394290, 7)Show InChI InChI=1S/C15H12FN3/c16-14-4-2-1-3-13(14)15-6-5-12(9-18-15)10-19-8-7-17-11-19/h1-9,11H,10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50396330
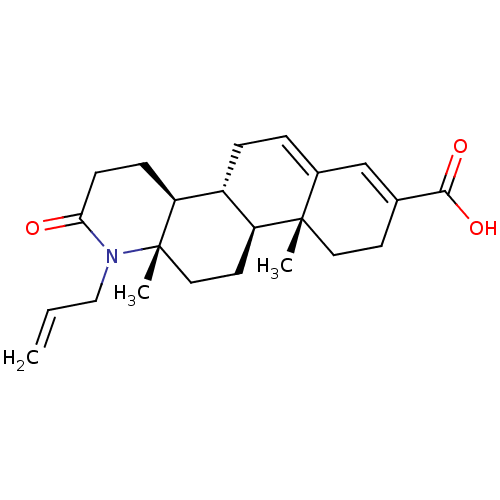
(CHEMBL2172645)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C=C(CC[C@]34C)C(O)=O)[C@@H]1CCC(=O)N2CC=C |r,c:9,t:7| Show InChI InChI=1S/C23H31NO3/c1-4-13-24-20(25)8-7-19-17-6-5-16-14-15(21(26)27)9-11-22(16,2)18(17)10-12-23(19,24)3/h4-5,14,17-19H,1,6-13H2,2-3H3,(H,26,27)/t17-,18+,19+,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of human type-2 5-alpha reductase expressed in HEK293 cells using [3H]-androstenedione as substrate after 30 mins by HPLC analysis |
Eur J Med Chem 54: 728-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.026
BindingDB Entry DOI: 10.7270/Q26T0NSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378947
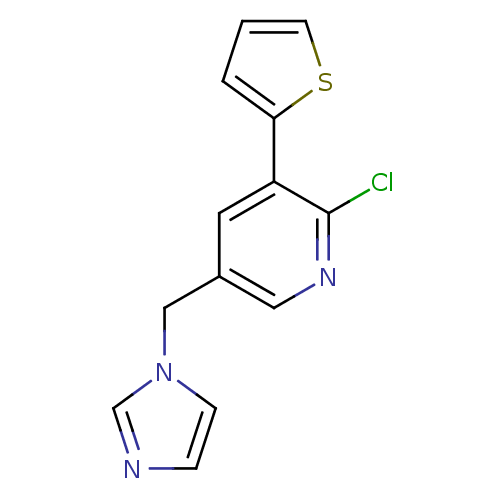
(CHEMBL2011430)Show InChI InChI=1S/C13H10ClN3S/c14-13-11(12-2-1-5-18-12)6-10(7-16-13)8-17-4-3-15-9-17/h1-7,9H,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378943
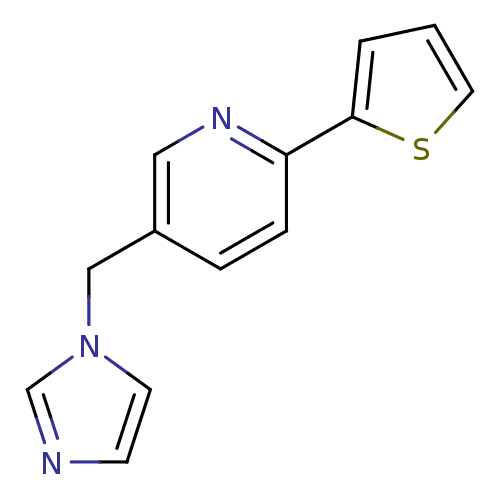
(CHEMBL2011261 | US9394290, 31)Show InChI InChI=1S/C13H11N3S/c1-2-13(17-7-1)12-4-3-11(8-15-12)9-16-6-5-14-10-16/h1-8,10H,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378952
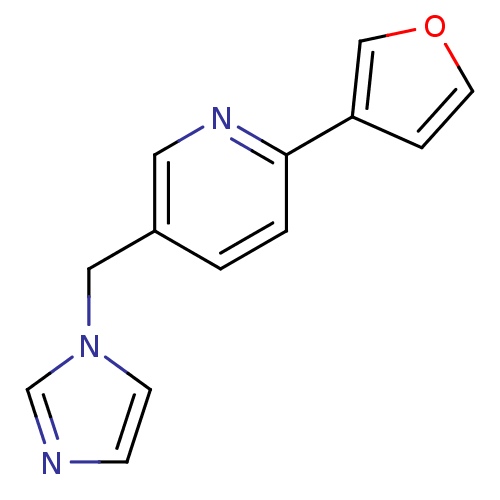
(CHEMBL2011435 | US9394290, 40)Show InChI InChI=1S/C13H11N3O/c1-2-13(12-3-6-17-9-12)15-7-11(1)8-16-5-4-14-10-16/h1-7,9-10H,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50141068
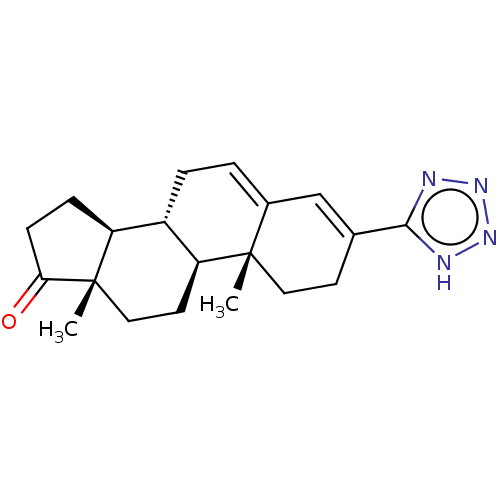
(CHEMBL3754525)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C=C(CC[C@]12C)c1nnn[nH]1 |r,c:19,t:17| Show InChI InChI=1S/C20H26N4O/c1-19-9-7-12(18-21-23-24-22-18)11-13(19)3-4-14-15-5-6-17(25)20(15,2)10-8-16(14)19/h3,11,14-16H,4-10H2,1-2H3,(H,21,22,23,24)/t14-,15-,16-,19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human 5alpha-2 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by... |
Bioorg Med Chem 24: 779-88 (2016)
Article DOI: 10.1016/j.bmc.2015.12.048
BindingDB Entry DOI: 10.7270/Q21G0P3C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50378942
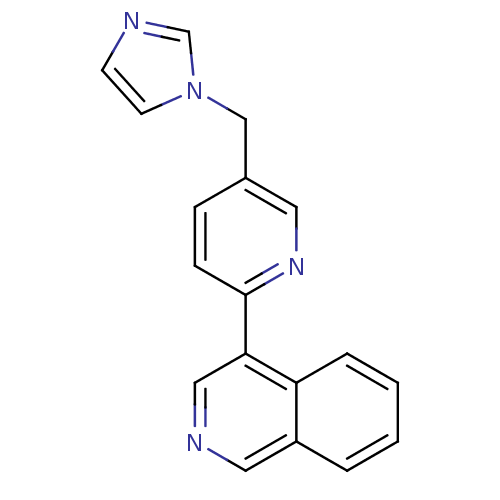
(CHEMBL2011260 | US9394290, 30)Show InChI InChI=1S/C18H14N4/c1-2-4-16-15(3-1)10-20-11-17(16)18-6-5-14(9-21-18)12-22-8-7-19-13-22/h1-11,13H,12H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79 MZ cells using [3H] 11 deoxycorticosterone as substrate by HPLC radioflow detector |
ACS Med Chem Lett 2: 559-564 (2011)
Article DOI: 10.1021/ml100283h
BindingDB Entry DOI: 10.7270/Q21R6RJ2 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322789

(4'-(pyridin-4-ylmethyl)biphenyl-3-ol | 4-[(3'-Hydr...)Show InChI InChI=1S/C18H15NO/c20-18-3-1-2-17(13-18)16-6-4-14(5-7-16)12-15-8-10-19-11-9-15/h1-11,13,20H,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
VIM-1 metallo-beta-lactamase
(Klebsiella pneumoniae) | BDBM50585867
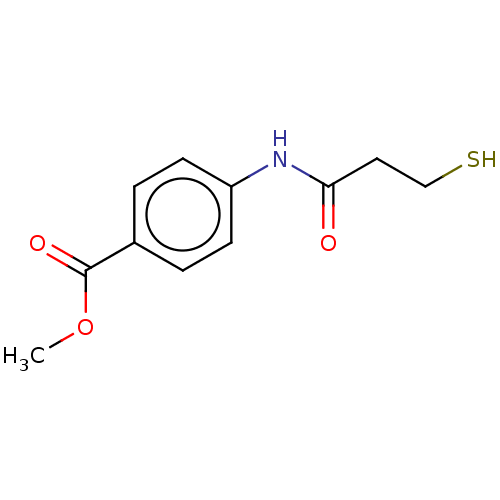
(CHEMBL5088797) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli VIM-1 using fluorocillin as substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01755
BindingDB Entry DOI: 10.7270/Q2QN6BP2 |
More data for this
Ligand-Target Pair | |
VIM-1 metallo-beta-lactamase
(Klebsiella pneumoniae) | BDBM50585864
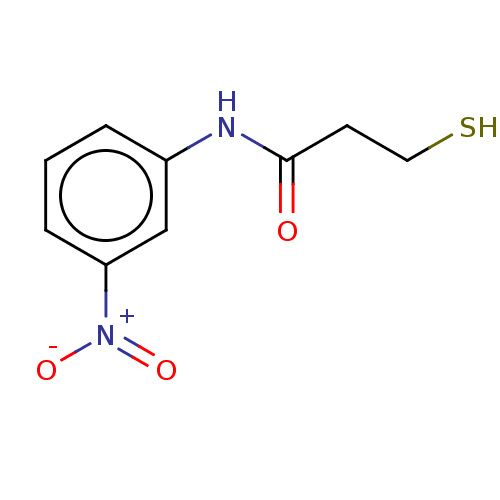
(CHEMBL5077613) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli VIM-1 using fluorocillin as substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01755
BindingDB Entry DOI: 10.7270/Q2QN6BP2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data