 Found 42 hits with Last Name = 'hayasaki-kajiwara' and Initial = 'y'
Found 42 hits with Last Name = 'hayasaki-kajiwara' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin G
(Homo sapiens (Human)) | BDBM50101132

(1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...)Show InChI InChI=1S/C16H14N2O2/c19-15-17(11-13-7-3-1-4-8-13)16(20)18(15)12-14-9-5-2-6-10-14/h1-10H,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human cathepsin G |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50101132

(1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...)Show InChI InChI=1S/C16H14N2O2/c19-15-17(11-13-7-3-1-4-8-13)16(20)18(15)12-14-9-5-2-6-10-14/h1-10H,11-12H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50101136

(1,3-Bis-benzo[1,3]dioxol-5-ylmethyl-[1,3]diazetidi...)Show InChI InChI=1S/C18H14N2O6/c21-17-19(7-11-1-3-13-15(5-11)25-9-23-13)18(22)20(17)8-12-2-4-14-16(6-12)26-10-24-14/h1-6H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human Serine protease chymase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50101135

(1,3-Bis-(4-methoxy-benzyl)-[1,3]diazetidine-2,4-di...)Show InChI InChI=1S/C18H18N2O4/c1-23-15-7-3-13(4-8-15)11-19-17(21)20(18(19)22)12-14-5-9-16(24-2)10-6-14/h3-10H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human Serine protease chymase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50101137
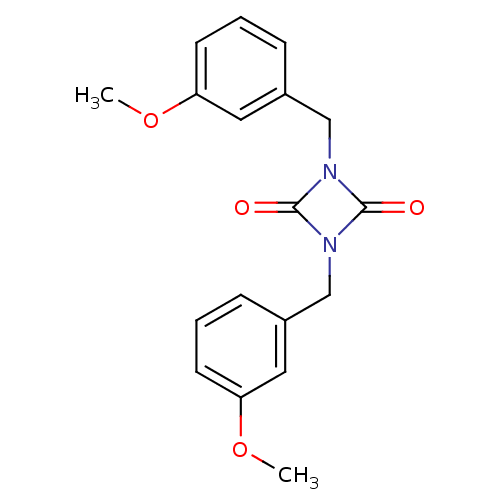
(1,3-Bis-(3-methoxy-benzyl)-[1,3]diazetidine-2,4-di...)Show InChI InChI=1S/C18H18N2O4/c1-23-15-7-3-5-13(9-15)11-19-17(21)20(18(19)22)12-14-6-4-8-16(10-14)24-2/h3-10H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human Serine protease chymase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50101131
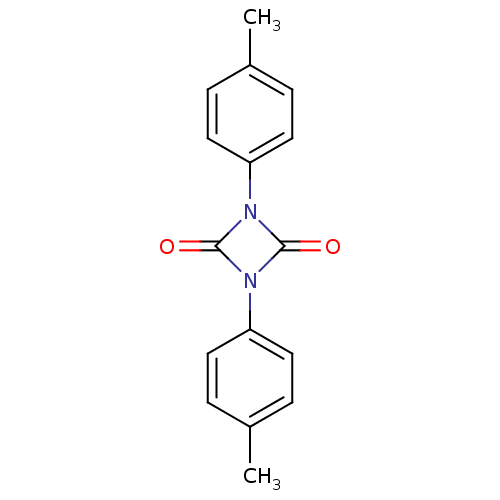
(1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...)Show InChI InChI=1S/C16H14N2O2/c1-11-3-7-13(8-4-11)17-15(19)18(16(17)20)14-9-5-12(2)6-10-14/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human cathepsin G |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50101131

(1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...)Show InChI InChI=1S/C16H14N2O2/c1-11-3-7-13(8-4-11)17-15(19)18(16(17)20)14-9-5-12(2)6-10-14/h3-10H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50101132

(1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...)Show InChI InChI=1S/C16H14N2O2/c19-15-17(11-13-7-3-1-4-8-13)16(20)18(15)12-14-9-5-2-6-10-14/h1-10H,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human Serine protease chymase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50101132

(1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...)Show InChI InChI=1S/C16H14N2O2/c19-15-17(11-13-7-3-1-4-8-13)16(20)18(15)12-14-9-5-2-6-10-14/h1-10H,11-12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against bovine pancreatic trypsin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50101134

(1,3-Bis-benzo[1,3]dioxol-5-yl-[1,3]diazetidine-2,4...)Show InChI InChI=1S/C16H10N2O6/c19-15-17(9-1-3-11-13(5-9)23-7-21-11)16(20)18(15)10-2-4-12-14(6-10)24-8-22-12/h1-6H,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human Serine protease chymase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50101133

(1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...)Show InChI InChI=1S/C6H10N2O2/c1-3-7-5(9)8(4-2)6(7)10/h3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human neutrophil elastase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101132

(1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...)Show InChI InChI=1S/C16H14N2O2/c19-15-17(11-13-7-3-1-4-8-13)16(20)18(15)12-14-9-5-2-6-10-14/h1-10H,11-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human thrombin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50101132

(1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...)Show InChI InChI=1S/C16H14N2O2/c19-15-17(11-13-7-3-1-4-8-13)16(20)18(15)12-14-9-5-2-6-10-14/h1-10H,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human neutrophil elastase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50101131

(1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...)Show InChI InChI=1S/C16H14N2O2/c1-11-3-7-13(8-4-11)17-15(19)18(16(17)20)14-9-5-12(2)6-10-14/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human Serine protease chymase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50101131

(1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...)Show InChI InChI=1S/C16H14N2O2/c1-11-3-7-13(8-4-11)17-15(19)18(16(17)20)14-9-5-12(2)6-10-14/h3-10H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against bovine pancreatic trypsin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50101131

(1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...)Show InChI InChI=1S/C16H14N2O2/c1-11-3-7-13(8-4-11)17-15(19)18(16(17)20)14-9-5-12(2)6-10-14/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human neutrophil elastase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50101133

(1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...)Show InChI InChI=1S/C6H10N2O2/c1-3-7-5(9)8(4-2)6(7)10/h3-4H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50101133

(1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...)Show InChI InChI=1S/C6H10N2O2/c1-3-7-5(9)8(4-2)6(7)10/h3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human cathepsin G |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101131

(1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...)Show InChI InChI=1S/C16H14N2O2/c1-11-3-7-13(8-4-11)17-15(19)18(16(17)20)14-9-5-12(2)6-10-14/h3-10H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human thrombin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50101132

(1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...)Show InChI InChI=1S/C16H14N2O2/c19-15-17(11-13-7-3-1-4-8-13)16(20)18(15)12-14-9-5-2-6-10-14/h1-10H,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human plasmin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50600525
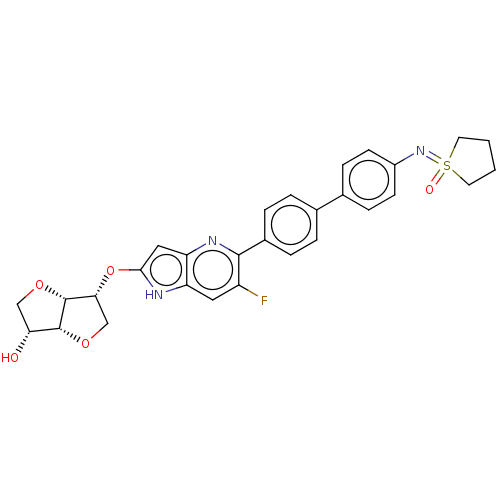
(CHEMBL5204493)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cc3)N=S3(=O)CCCC3)[C@@]1([H])OC[C@H]2O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101133

(1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...)Show InChI InChI=1S/C6H10N2O2/c1-3-7-5(9)8(4-2)6(7)10/h3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human thrombin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50600523
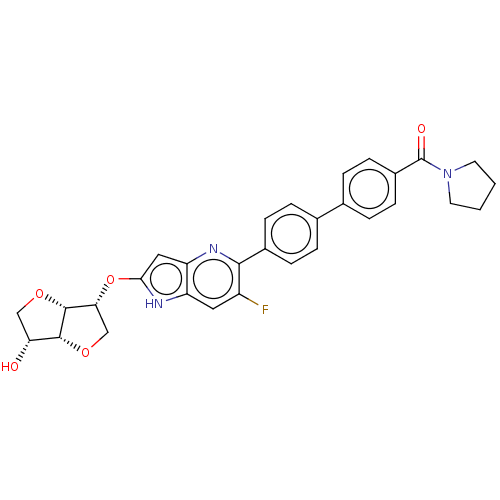
(CHEMBL5184386)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cc3)C(=O)N3CCCC3)[C@@]1([H])OC[C@H]2O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50600524
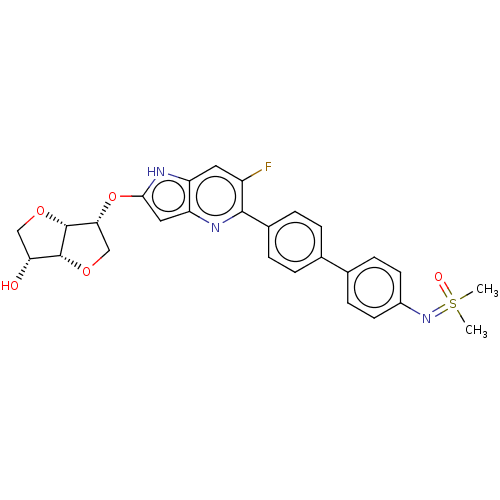
(CHEMBL5177715)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cc3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50101133

(1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...)Show InChI InChI=1S/C6H10N2O2/c1-3-7-5(9)8(4-2)6(7)10/h3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human plasmin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50600526
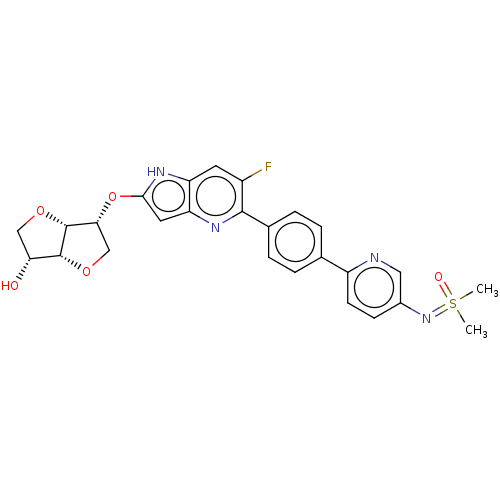
(CHEMBL5188431)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cn3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50600526

(CHEMBL5188431)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cn3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50600524

(CHEMBL5177715)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cc3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50600526

(CHEMBL5188431)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cn3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50600524

(CHEMBL5177715)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cc3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50600526

(CHEMBL5188431)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cn3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50600524

(CHEMBL5177715)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cc3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50600526

(CHEMBL5188431)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cn3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50600524

(CHEMBL5177715)Show SMILES [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cc3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50405588

(CHEMBL5288610)Show SMILES NCCCC[C@H](OP(O)(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C19H29N2O6P/c20-12-5-4-10-17(18(22)21-13-6-9-16(21)19(23)24)27-28(25,26)14-11-15-7-2-1-3-8-15/h1-3,7-8,16-17H,4-6,9-14,20H2,(H,23,24)(H,25,26)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50405588

(CHEMBL5288610)Show SMILES NCCCC[C@H](OP(O)(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C19H29N2O6P/c20-12-5-4-10-17(18(22)21-13-6-9-16(21)19(23)24)27-28(25,26)14-11-15-7-2-1-3-8-15/h1-3,7-8,16-17H,4-6,9-14,20H2,(H,23,24)(H,25,26)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50405588

(CHEMBL5288610)Show SMILES NCCCC[C@H](OP(O)(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C19H29N2O6P/c20-12-5-4-10-17(18(22)21-13-6-9-16(21)19(23)24)27-28(25,26)14-11-15-7-2-1-3-8-15/h1-3,7-8,16-17H,4-6,9-14,20H2,(H,23,24)(H,25,26)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50405588

(CHEMBL5288610)Show SMILES NCCCC[C@H](OP(O)(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C19H29N2O6P/c20-12-5-4-10-17(18(22)21-13-6-9-16(21)19(23)24)27-28(25,26)14-11-15-7-2-1-3-8-15/h1-3,7-8,16-17H,4-6,9-14,20H2,(H,23,24)(H,25,26)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50405588

(CHEMBL5288610)Show SMILES NCCCC[C@H](OP(O)(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C19H29N2O6P/c20-12-5-4-10-17(18(22)21-13-6-9-16(21)19(23)24)27-28(25,26)14-11-15-7-2-1-3-8-15/h1-3,7-8,16-17H,4-6,9-14,20H2,(H,23,24)(H,25,26)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50101133

(1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...)Show InChI InChI=1S/C6H10N2O2/c1-3-7-5(9)8(4-2)6(7)10/h3-4H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against bovine pancreatic trypsin |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase subunit beta-2
(Homo sapiens (Human)) | BDBM50600528
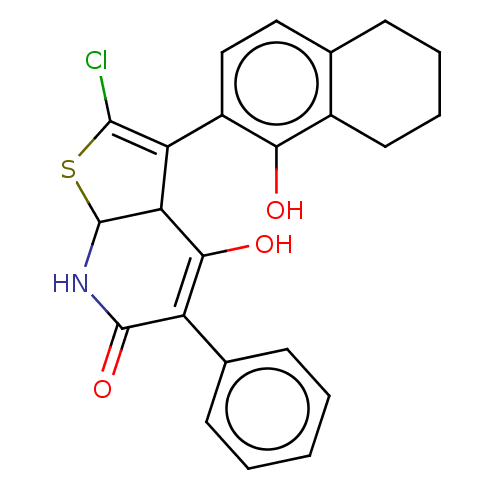
(CHEMBL5191686)Show SMILES OC1=C(C(=O)NC2SC(Cl)=C(C12)c1ccc2CCCCc2c1O)c1ccccc1 |c:9,t:1| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase subunit beta-1
(Rattus norvegicus) | BDBM50600527

(CHEMBL5201587)Show SMILES OC1=C(C#N)C(=O)NC2SC=C(C12)c1ccc(cc1)-c1ccccc1O |c:1,10| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128769
BindingDB Entry DOI: 10.7270/Q2DJ5KPR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data