| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50600526 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2234460 (CHEMBL5148232) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Tamura, Y; Morita, I; Hinata, Y; Kojima, E; Ozasa, H; Ikemoto, H; Asano, M; Wada, T; Hayasaki-Kajiwara, Y; Iwasaki, T; Matsumura, K Identification of novel indole derivatives as highly potent AMPK activators with anti-diabetic profiles. Bioorg Med Chem Lett68:0 (2022) [PubMed] Article Tamura, Y; Morita, I; Hinata, Y; Kojima, E; Ozasa, H; Ikemoto, H; Asano, M; Wada, T; Hayasaki-Kajiwara, Y; Iwasaki, T; Matsumura, K Identification of novel indole derivatives as highly potent AMPK activators with anti-diabetic profiles. Bioorg Med Chem Lett68:0 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50600526 |
|---|
| n/a |
|---|
| Name | BDBM50600526 |
|---|
| Synonyms: | CHEMBL5188431 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H25FN4O5S |
|---|
| Mol. Mass. | 524.564 |
|---|
| SMILES | [H][C@]12OC[C@@H](Oc3cc4nc(c(F)cc4[nH]3)-c3ccc(cc3)-c3ccc(cn3)N=S(C)(C)=O)[C@@]1([H])OC[C@H]2O |r| |
|---|
| Structure | 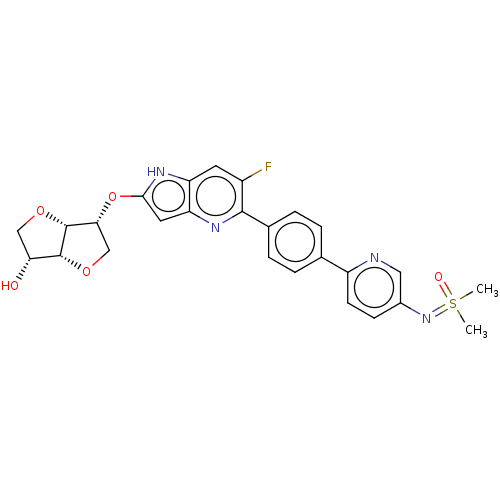
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tamura, Y; Morita, I; Hinata, Y; Kojima, E; Ozasa, H; Ikemoto, H; Asano, M; Wada, T; Hayasaki-Kajiwara, Y; Iwasaki, T; Matsumura, K Identification of novel indole derivatives as highly potent AMPK activators with anti-diabetic profiles. Bioorg Med Chem Lett68:0 (2022) [PubMed] Article
Tamura, Y; Morita, I; Hinata, Y; Kojima, E; Ozasa, H; Ikemoto, H; Asano, M; Wada, T; Hayasaki-Kajiwara, Y; Iwasaki, T; Matsumura, K Identification of novel indole derivatives as highly potent AMPK activators with anti-diabetic profiles. Bioorg Med Chem Lett68:0 (2022) [PubMed] Article