 Found 245 hits with Last Name = 'henning' and Initial = 'r'
Found 245 hits with Last Name = 'henning' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM50019848
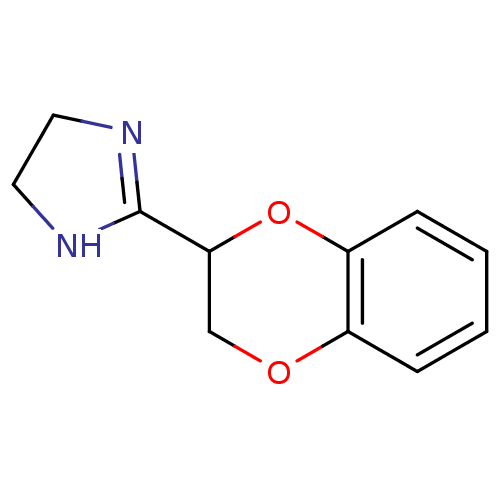
(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019719
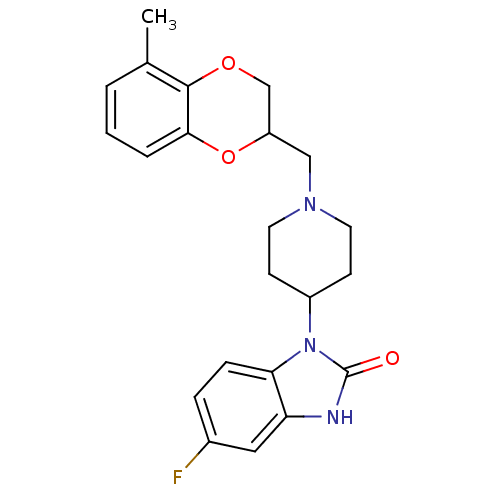
(5-Fluoro-1-[1-(5-methyl-2,3-dihydro-benzo[1,4]diox...)Show SMILES Cc1cccc2OC(CN3CCC(CC3)n3c4ccc(F)cc4[nH]c3=O)COc12 Show InChI InChI=1S/C22H24FN3O3/c1-14-3-2-4-20-21(14)28-13-17(29-20)12-25-9-7-16(8-10-25)26-19-6-5-15(23)11-18(19)24-22(26)27/h2-6,11,16-17H,7-10,12-13H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards dopamine receptor D2 was determined by displacement of [3H]-spiroperidol from bovine nucleus caudate membranes. |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019721
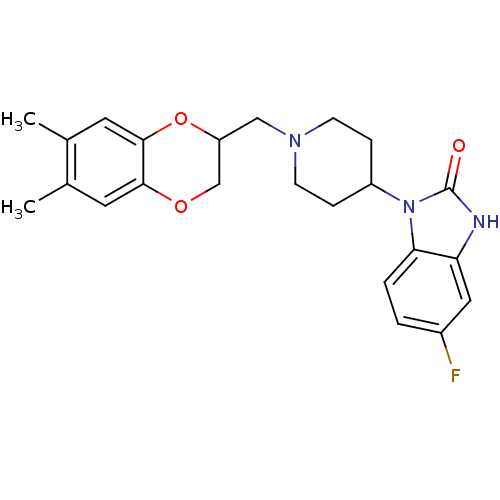
(1-[1-(6,7-Dimethyl-2,3-dihydro-benzo[1,4]dioxin-2-...)Show SMILES Cc1cc2OCC(CN3CCC(CC3)n3c4ccc(F)cc4[nH]c3=O)Oc2cc1C Show InChI InChI=1S/C23H26FN3O3/c1-14-9-21-22(10-15(14)2)30-18(13-29-21)12-26-7-5-17(6-8-26)27-20-4-3-16(24)11-19(20)25-23(27)28/h3-4,9-11,17-18H,5-8,12-13H2,1-2H3,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019726
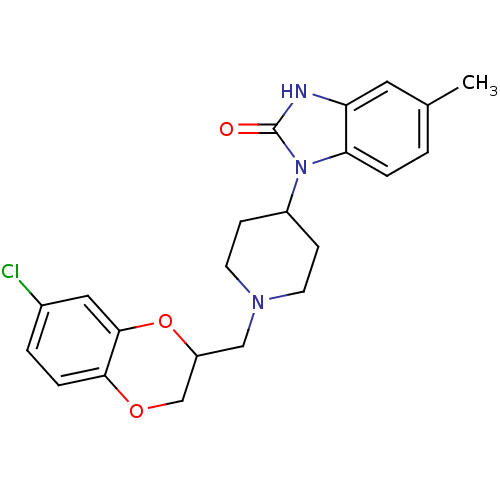
(1-[1-(7-Chloro-2,3-dihydro-benzo[1,4]dioxin-2-ylme...)Show SMILES Cc1ccc2n(C3CCN(CC4COc5ccc(Cl)cc5O4)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H24ClN3O3/c1-14-2-4-19-18(10-14)24-22(27)26(19)16-6-8-25(9-7-16)12-17-13-28-20-5-3-15(23)11-21(20)29-17/h2-5,10-11,16-17H,6-9,12-13H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards dopamine receptor D2 was determined by displacement of [3H]-spiroperidol from bovine nucleus caudate membranes. |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019717

(5-Chloro-1-[1-(2,3-dihydro-benzo[1,4]dioxin-2-ylme...)Show SMILES Clc1ccc2n(C3CCN(CC4COc5ccccc5O4)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C21H22ClN3O3/c22-14-5-6-18-17(11-14)23-21(26)25(18)15-7-9-24(10-8-15)12-16-13-27-19-3-1-2-4-20(19)28-16/h1-6,11,15-16H,7-10,12-13H2,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM35256
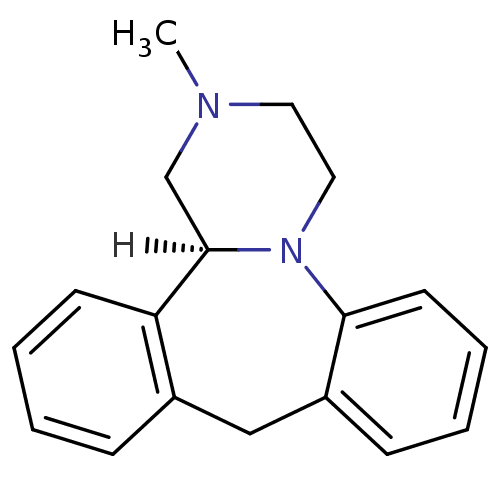
((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019725
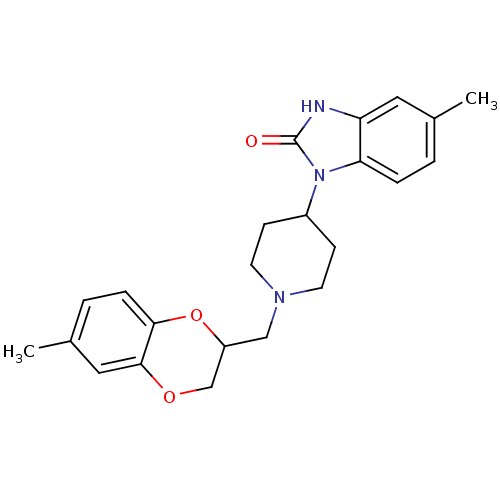
(5-Methyl-1-[1-(6-methyl-2,3-dihydro-benzo[1,4]diox...)Show SMILES Cc1ccc2OC(CN3CCC(CC3)n3c4ccc(C)cc4[nH]c3=O)COc2c1 Show InChI InChI=1S/C23H27N3O3/c1-15-3-5-20-19(11-15)24-23(27)26(20)17-7-9-25(10-8-17)13-18-14-28-22-12-16(2)4-6-21(22)29-18/h3-6,11-12,17-18H,7-10,13-14H2,1-2H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019727

(1-[1-(6,7-Dimethyl-2,3-dihydro-benzo[1,4]dioxin-2-...)Show SMILES Cc1ccc2n(C3CCN(CC4COc5cc(C)c(C)cc5O4)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C24H29N3O3/c1-15-4-5-21-20(10-15)25-24(28)27(21)18-6-8-26(9-7-18)13-19-14-29-22-11-16(2)17(3)12-23(22)30-19/h4-5,10-12,18-19H,6-9,13-14H2,1-3H3,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM50016897
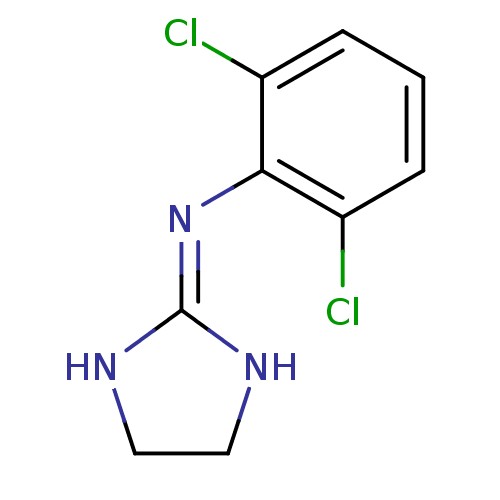
(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019724

(5-Chloro-1-[1-(6-methyl-2,3-dihydro-benzo[1,4]diox...)Show SMILES Cc1ccc2OC(CN3CCC(CC3)n3c4ccc(Cl)cc4[nH]c3=O)COc2c1 Show InChI InChI=1S/C22H24ClN3O3/c1-14-2-5-20-21(10-14)28-13-17(29-20)12-25-8-6-16(7-9-25)26-19-4-3-15(23)11-18(19)24-22(26)27/h2-5,10-11,16-17H,6-9,12-13H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019728
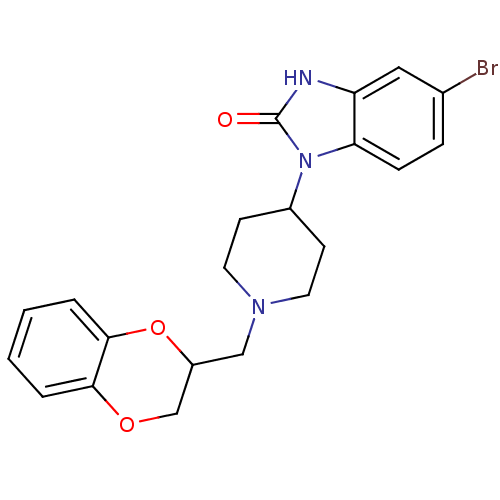
(5-Bromo-1-[1-(2,3-dihydro-benzo[1,4]dioxin-2-ylmet...)Show SMILES Brc1ccc2n(C3CCN(CC4COc5ccccc5O4)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C21H22BrN3O3/c22-14-5-6-18-17(11-14)23-21(26)25(18)15-7-9-24(10-8-15)12-16-13-27-19-3-1-2-4-20(19)28-16/h1-6,11,15-16H,7-10,12-13H2,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019718

(5-Fluoro-1-[1-(6-fluoro-2,3-dihydro-benzo[1,4]diox...)Show SMILES Fc1ccc2OC(CN3CCC(CC3)n3c4ccc(F)cc4[nH]c3=O)COc2c1 Show InChI InChI=1S/C21H21F2N3O3/c22-13-1-3-18-17(9-13)24-21(27)26(18)15-5-7-25(8-6-15)11-16-12-28-20-10-14(23)2-4-19(20)29-16/h1-4,9-10,15-16H,5-8,11-12H2,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019723

(1-[1-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-pip...)Show SMILES Cc1ccc2n(C3CCN(CC4COc5ccccc5O4)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H25N3O3/c1-15-6-7-19-18(12-15)23-22(26)25(19)16-8-10-24(11-9-16)13-17-14-27-20-4-2-3-5-21(20)28-17/h2-7,12,16-17H,8-11,13-14H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019722
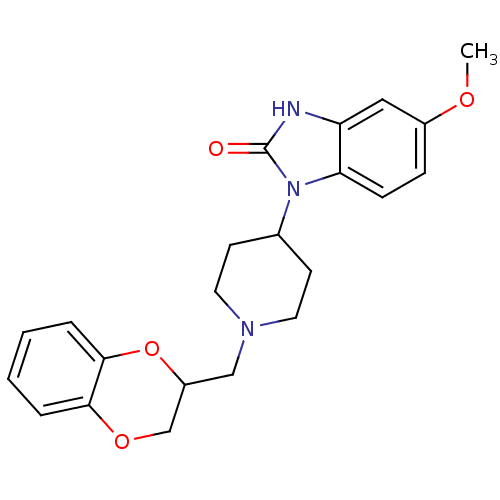
(1-[1-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-pip...)Show SMILES COc1ccc2n(C3CCN(CC4COc5ccccc5O4)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H25N3O4/c1-27-16-6-7-19-18(12-16)23-22(26)25(19)15-8-10-24(11-9-15)13-17-14-28-20-4-2-3-5-21(20)29-17/h2-7,12,15,17H,8-11,13-14H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50019720
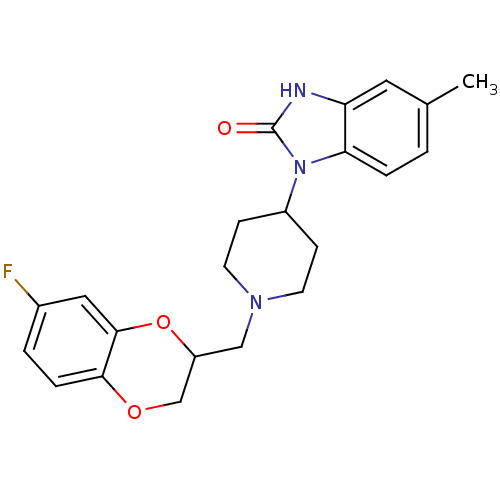
(1-[1-(7-Fluoro-2,3-dihydro-benzo[1,4]dioxin-2-ylme...)Show SMILES Cc1ccc2n(C3CCN(CC4COc5ccc(F)cc5O4)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H24FN3O3/c1-14-2-4-19-18(10-14)24-22(27)26(19)16-6-8-25(9-7-16)12-17-13-28-20-5-3-15(23)11-21(20)29-17/h2-5,10-11,16-17H,6-9,12-13H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 |
J Med Chem 30: 814-9 (1987)
BindingDB Entry DOI: 10.7270/Q2PR7TZJ |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM35256

((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50016897

(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM81926
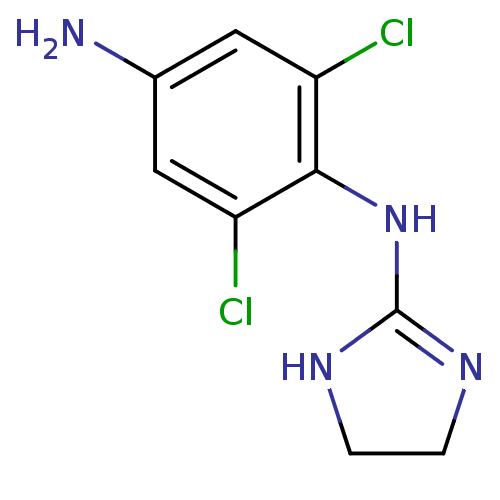
(Aminoclonidine, p | CAS_66711-21-5 | NSC_51763 | p...)Show InChI InChI=1S/C9H10Cl2N4/c10-6-3-5(12)4-7(11)8(6)15-9-13-1-2-14-9/h3-4H,1-2,12H2,(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019848

(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1108-16 (1990)
BindingDB Entry DOI: 10.7270/Q2TD9VV5 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045686
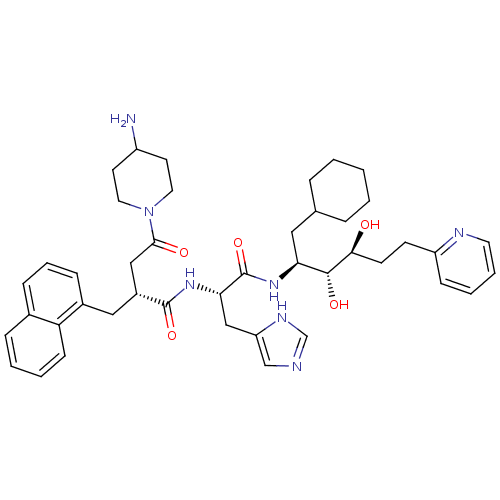
(4-(4-Amino-piperidin-1-yl)-N-[1-(1-cyclohexylmethy...)Show SMILES NC1CCN(CC1)C(=O)C[C@@H](Cc1cccc2ccccc12)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C43H57N7O5/c44-33-18-21-50(22-19-33)40(52)25-32(24-31-13-8-12-30-11-4-5-15-36(30)31)42(54)49-38(26-35-27-45-28-47-35)43(55)48-37(23-29-9-2-1-3-10-29)41(53)39(51)17-16-34-14-6-7-20-46-34/h4-8,11-15,20,27-29,32-33,37-39,41,51,53H,1-3,9-10,16-19,21-26,44H2,(H,45,47)(H,48,55)(H,49,54)/t32-,37+,38+,39+,41-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human kidney renin |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045691
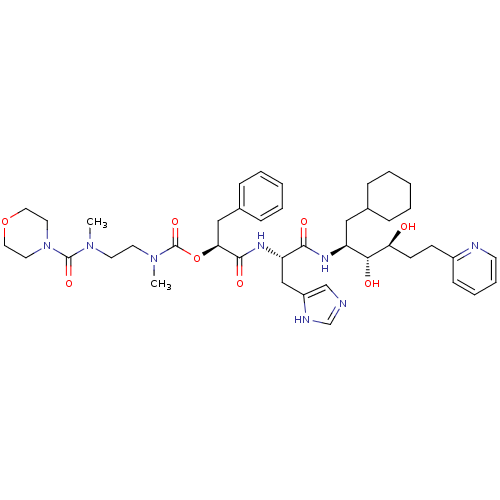
(CHEMBL94827 | Methyl-{2-[methyl-(morpholine-4-carb...)Show SMILES CN(CCN(C)C(=O)N1CCOCC1)C(=O)O[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C42H60N8O8/c1-48(41(55)50-21-23-57-24-22-50)19-20-49(2)42(56)58-37(26-31-13-7-4-8-14-31)40(54)47-35(27-33-28-43-29-45-33)39(53)46-34(25-30-11-5-3-6-12-30)38(52)36(51)17-16-32-15-9-10-18-44-32/h4,7-10,13-15,18,28-30,34-38,51-52H,3,5-6,11-12,16-17,19-27H2,1-2H3,(H,43,45)(H,46,53)(H,47,54)/t34-,35-,36-,37-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human kidney renin |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045691

(CHEMBL94827 | Methyl-{2-[methyl-(morpholine-4-carb...)Show SMILES CN(CCN(C)C(=O)N1CCOCC1)C(=O)O[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C42H60N8O8/c1-48(41(55)50-21-23-57-24-22-50)19-20-49(2)42(56)58-37(26-31-13-7-4-8-14-31)40(54)47-35(27-33-28-43-29-45-33)39(53)46-34(25-30-11-5-3-6-12-30)38(52)36(51)17-16-32-15-9-10-18-44-32/h4,7-10,13-15,18,28-30,34-38,51-52H,3,5-6,11-12,16-17,19-27H2,1-2H3,(H,43,45)(H,46,53)(H,47,54)/t34-,35-,36-,37-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of renin in human plasma |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045687
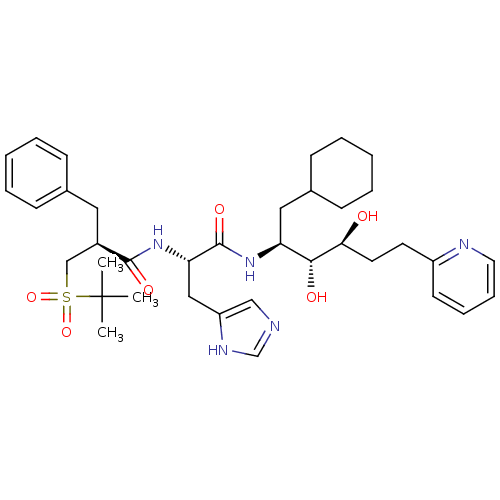
(2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C37H53N5O6S/c1-37(2,3)49(47,48)24-28(20-26-12-6-4-7-13-26)35(45)42-32(22-30-23-38-25-40-30)36(46)41-31(21-27-14-8-5-9-15-27)34(44)33(43)18-17-29-16-10-11-19-39-29/h4,6-7,10-13,16,19,23,25,27-28,31-34,43-44H,5,8-9,14-15,17-18,20-22,24H2,1-3H3,(H,38,40)(H,41,46)(H,42,45)/t28-,31+,32+,33+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of renin in human plasma |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50045683
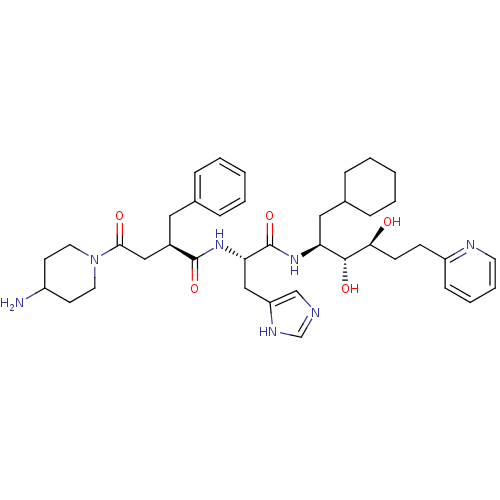
(4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...)Show SMILES NC1CCN(CC1)C(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C39H55N7O5/c40-30-16-19-46(20-17-30)36(48)23-29(21-27-9-3-1-4-10-27)38(50)45-34(24-32-25-41-26-43-32)39(51)44-33(22-28-11-5-2-6-12-28)37(49)35(47)15-14-31-13-7-8-18-42-31/h1,3-4,7-10,13,18,25-26,28-30,33-35,37,47,49H,2,5-6,11-12,14-17,19-24,40H2,(H,41,43)(H,44,51)(H,45,50)/t29-,33+,34+,35+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of renin in human plasma |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045685
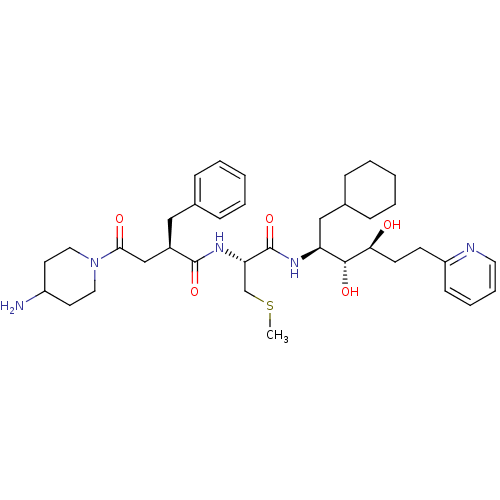
(4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...)Show SMILES CSC[C@H](NC(=O)[C@@H](CC(=O)N1CCC(N)CC1)Cc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C37H55N5O5S/c1-48-25-32(41-36(46)28(22-26-10-4-2-5-11-26)24-34(44)42-20-17-29(38)18-21-42)37(47)40-31(23-27-12-6-3-7-13-27)35(45)33(43)16-15-30-14-8-9-19-39-30/h2,4-5,8-11,14,19,27-29,31-33,35,43,45H,3,6-7,12-13,15-18,20-25,38H2,1H3,(H,40,47)(H,41,46)/t28-,31+,32+,33+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human kidney renin |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045685

(4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...)Show SMILES CSC[C@H](NC(=O)[C@@H](CC(=O)N1CCC(N)CC1)Cc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C37H55N5O5S/c1-48-25-32(41-36(46)28(22-26-10-4-2-5-11-26)24-34(44)42-20-17-29(38)18-21-42)37(47)40-31(23-27-12-6-3-7-13-27)35(45)33(43)16-15-30-14-8-9-19-39-30/h2,4-5,8-11,14,19,27-29,31-33,35,43,45H,3,6-7,12-13,15-18,20-25,38H2,1H3,(H,40,47)(H,41,46)/t28-,31+,32+,33+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human kidney renin |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426601
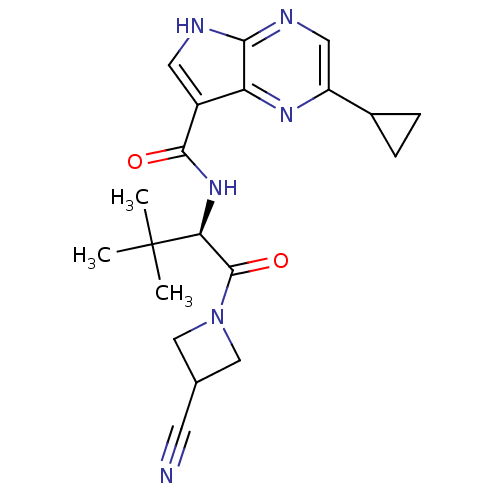
(CHEMBL2325895)Show SMILES CC(C)(C)[C@@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C20H24N6O2/c1-20(2,3)16(19(28)26-9-11(6-21)10-26)25-18(27)13-7-22-17-15(13)24-14(8-23-17)12-4-5-12/h7-8,11-12,16H,4-5,9-10H2,1-3H3,(H,22,23)(H,25,27)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045692
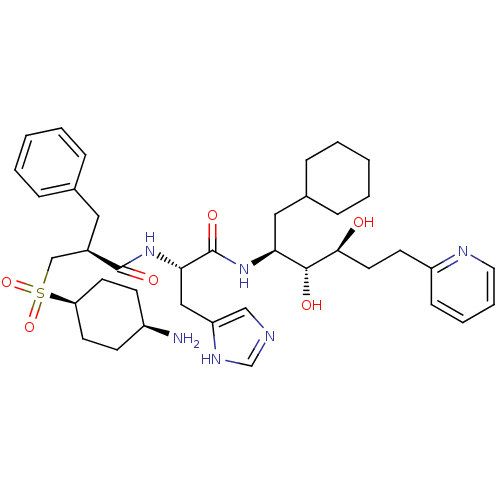
(3-(4-Amino-cyclohexanesulfonyl)-2-benzyl-N-[1-(1-c...)Show SMILES N[C@H]1CC[C@H](CC1)S(=O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 |wU:11.11,32.34,4.7,1.0,42.46,wD:22.23,40.44,(-.88,-15.35,;.47,-14.59,;.5,-13.05,;1.83,-12.3,;3.16,-13.09,;3.13,-14.63,;1.8,-15.38,;4.49,-12.33,;3.39,-11.23,;5.58,-11.23,;5.82,-13.11,;7.17,-12.34,;7.17,-10.8,;6.4,-9.47,;7.17,-8.12,;6.38,-6.77,;4.84,-6.79,;4.07,-8.14,;4.84,-9.47,;8.5,-13.12,;8.48,-14.66,;9.83,-12.37,;11.16,-13.14,;11.14,-14.68,;12.47,-15.45,;13.89,-14.84,;14.92,-15.99,;14.12,-17.32,;12.63,-16.99,;12.49,-12.37,;12.51,-10.83,;13.82,-13.14,;15.17,-12.39,;15.17,-10.85,;15.94,-9.52,;15.17,-8.17,;15.94,-6.84,;17.48,-6.86,;18.25,-8.17,;17.48,-9.52,;16.5,-13.16,;16.48,-14.7,;17.83,-12.41,;17.83,-10.87,;19.16,-13.18,;20.49,-12.42,;21.82,-13.21,;21.82,-14.75,;23.15,-15.52,;24.48,-14.77,;24.48,-13.21,;23.15,-12.44,)| Show InChI InChI=1S/C39H56N6O6S/c40-30-14-17-33(18-15-30)52(50,51)25-29(21-27-9-3-1-4-10-27)38(48)45-35(23-32-24-41-26-43-32)39(49)44-34(22-28-11-5-2-6-12-28)37(47)36(46)19-16-31-13-7-8-20-42-31/h1,3-4,7-10,13,20,24,26,28-30,33-37,46-47H,2,5-6,11-12,14-19,21-23,25,40H2,(H,41,43)(H,44,49)(H,45,48)/t29-,30-,33+,34+,35+,36+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human kidney renin |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50045692

(3-(4-Amino-cyclohexanesulfonyl)-2-benzyl-N-[1-(1-c...)Show SMILES N[C@H]1CC[C@H](CC1)S(=O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 |wU:11.11,32.34,4.7,1.0,42.46,wD:22.23,40.44,(-.88,-15.35,;.47,-14.59,;.5,-13.05,;1.83,-12.3,;3.16,-13.09,;3.13,-14.63,;1.8,-15.38,;4.49,-12.33,;3.39,-11.23,;5.58,-11.23,;5.82,-13.11,;7.17,-12.34,;7.17,-10.8,;6.4,-9.47,;7.17,-8.12,;6.38,-6.77,;4.84,-6.79,;4.07,-8.14,;4.84,-9.47,;8.5,-13.12,;8.48,-14.66,;9.83,-12.37,;11.16,-13.14,;11.14,-14.68,;12.47,-15.45,;13.89,-14.84,;14.92,-15.99,;14.12,-17.32,;12.63,-16.99,;12.49,-12.37,;12.51,-10.83,;13.82,-13.14,;15.17,-12.39,;15.17,-10.85,;15.94,-9.52,;15.17,-8.17,;15.94,-6.84,;17.48,-6.86,;18.25,-8.17,;17.48,-9.52,;16.5,-13.16,;16.48,-14.7,;17.83,-12.41,;17.83,-10.87,;19.16,-13.18,;20.49,-12.42,;21.82,-13.21,;21.82,-14.75,;23.15,-15.52,;24.48,-14.77,;24.48,-13.21,;23.15,-12.44,)| Show InChI InChI=1S/C39H56N6O6S/c40-30-14-17-33(18-15-30)52(50,51)25-29(21-27-9-3-1-4-10-27)38(48)45-35(23-32-24-41-26-43-32)39(49)44-34(22-28-11-5-2-6-12-28)37(47)36(46)19-16-31-13-7-8-20-42-31/h1,3-4,7-10,13,20,24,26,28-30,33-37,46-47H,2,5-6,11-12,14-19,21-23,25,40H2,(H,41,43)(H,44,49)(H,45,48)/t29-,30-,33+,34+,35+,36+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human kidney renin |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50045693

(4-Amino-piperidine-1-carboxylic acid 1-[1-(1-cyclo...)Show SMILES NC1CCN(CC1)C(=O)O[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C38H53N7O6/c39-28-16-19-45(20-17-28)38(50)51-34(22-27-11-5-2-6-12-27)37(49)44-32(23-30-24-40-25-42-30)36(48)43-31(21-26-9-3-1-4-10-26)35(47)33(46)15-14-29-13-7-8-18-41-29/h2,5-8,11-13,18,24-26,28,31-35,46-47H,1,3-4,9-10,14-17,19-23,39H2,(H,40,42)(H,43,48)(H,44,49)/t31-,32-,33-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of renin in human plasma |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045693

(4-Amino-piperidine-1-carboxylic acid 1-[1-(1-cyclo...)Show SMILES NC1CCN(CC1)C(=O)O[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C38H53N7O6/c39-28-16-19-45(20-17-28)38(50)51-34(22-27-11-5-2-6-12-27)37(49)44-32(23-30-24-40-25-42-30)36(48)43-31(21-26-9-3-1-4-10-26)35(47)33(46)15-14-29-13-7-8-18-41-29/h2,5-8,11-13,18,24-26,28,31-35,46-47H,1,3-4,9-10,14-17,19-23,39H2,(H,40,42)(H,43,48)(H,44,49)/t31-,32-,33-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of renin in human plasma |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045683

(4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...)Show SMILES NC1CCN(CC1)C(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C39H55N7O5/c40-30-16-19-46(20-17-30)36(48)23-29(21-27-9-3-1-4-10-27)38(50)45-34(24-32-25-41-26-43-32)39(51)44-33(22-28-11-5-2-6-12-28)37(49)35(47)15-14-31-13-7-8-18-42-31/h1,3-4,7-10,13,18,25-26,28-30,33-35,37,47,49H,2,5-6,11-12,14-17,19-24,40H2,(H,41,43)(H,44,51)(H,45,50)/t29-,33+,34+,35+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human kidney renin |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045683

(4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...)Show SMILES NC1CCN(CC1)C(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C39H55N7O5/c40-30-16-19-46(20-17-30)36(48)23-29(21-27-9-3-1-4-10-27)38(50)45-34(24-32-25-41-26-43-32)39(51)44-33(22-28-11-5-2-6-12-28)37(49)35(47)15-14-31-13-7-8-18-42-31/h1,3-4,7-10,13,18,25-26,28-30,33-35,37,47,49H,2,5-6,11-12,14-17,19-24,40H2,(H,41,43)(H,44,51)(H,45,50)/t29-,33+,34+,35+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human kidney renin |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50045687

(2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CCc1ccccn1 Show InChI InChI=1S/C37H53N5O6S/c1-37(2,3)49(47,48)24-28(20-26-12-6-4-7-13-26)35(45)42-32(22-30-23-38-25-40-30)36(46)41-31(21-27-14-8-5-9-15-27)34(44)33(43)18-17-29-16-10-11-19-39-29/h4,6-7,10-13,16,19,23,25,27-28,31-34,43-44H,5,8-9,14-15,17-18,20-22,24H2,1-3H3,(H,38,40)(H,41,46)(H,42,45)/t28-,31+,32+,33+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of renin in human plasma |
J Med Chem 36: 2788-800 (1993)
BindingDB Entry DOI: 10.7270/Q24J0D61 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426599
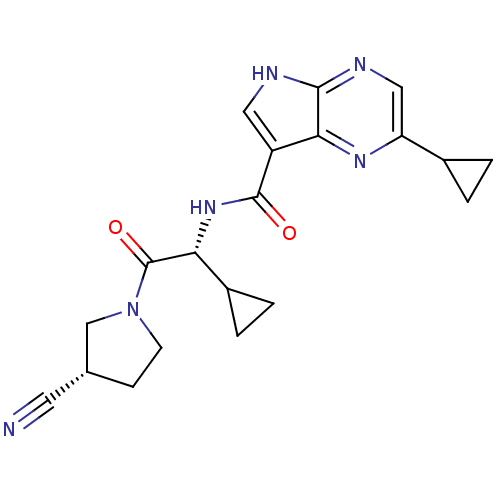
(CHEMBL2325898)Show SMILES O=C(N[C@H](C1CC1)C(=O)N1CC[C@@H](C1)C#N)c1c[nH]c2ncc(nc12)C1CC1 |r| Show InChI InChI=1S/C20H22N6O2/c21-7-11-5-6-26(10-11)20(28)16(13-3-4-13)25-19(27)14-8-22-18-17(14)24-15(9-23-18)12-1-2-12/h8-9,11-13,16H,1-6,10H2,(H,22,23)(H,25,27)/t11-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data