 Found 32 hits with Last Name = 'henwood' and Initial = 'a'
Found 32 hits with Last Name = 'henwood' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386376

(CHEMBL2046865)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6](-c1ccccc1)-c1ccccc1)-[#6](=O)-[#6]-[#6]-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C29H34N4O3/c30-29(31)32-20-8-7-13-25(26(35)19-16-21-14-17-24(34)18-15-21)33-28(36)27(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-6,9-12,14-15,17-18,25,27,34H,7-8,13,16,19-20H2,(H,33,36)(H4,30,31,32)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50123474
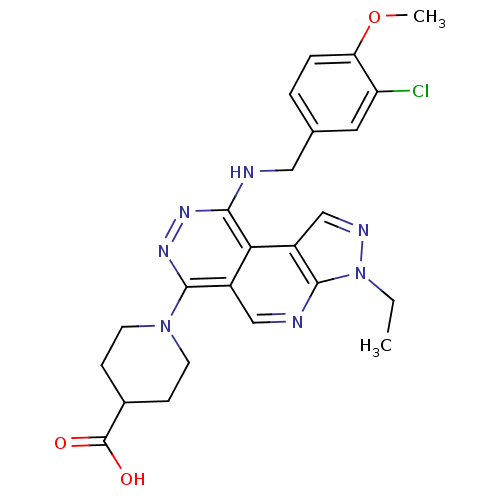
(1-[9-(3-Chloro-4-methoxy-benzylamino)-3-ethyl-3H-2...)Show SMILES CCn1ncc2c1ncc1c(nnc(NCc3ccc(OC)c(Cl)c3)c21)N1CCC(CC1)C(O)=O Show InChI InChI=1S/C24H26ClN7O3/c1-3-32-22-17(13-28-32)20-16(12-27-22)23(31-8-6-15(7-9-31)24(33)34)30-29-21(20)26-11-14-4-5-19(35-2)18(25)10-14/h4-5,10,12-13,15H,3,6-9,11H2,1-2H3,(H,26,29)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50123477

(CHEMBL344018 | N*9*-(3-Chloro-4-methoxy-benzyl)-3-...)Show SMILES CCn1ncc2c1ncc1c(NCc3ccncc3)nnc(NCc3ccc(OC)c(Cl)c3)c21 Show InChI InChI=1S/C24H23ClN8O/c1-3-33-24-18(14-30-33)21-17(13-29-24)22(27-11-15-6-8-26-9-7-15)31-32-23(21)28-12-16-4-5-20(34-2)19(25)10-16/h4-10,13-14H,3,11-12H2,1-2H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50123473
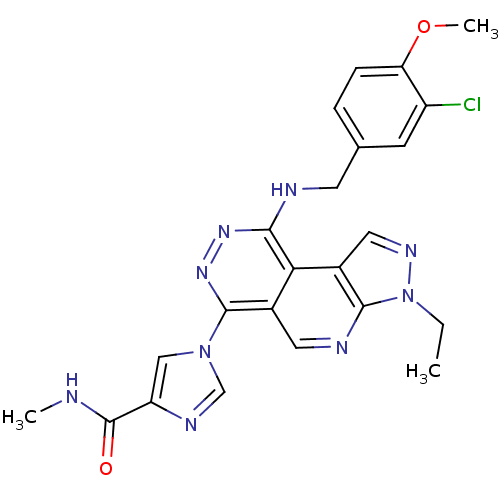
(1-[9-(3-Chloro-4-methoxy-benzylamino)-3-ethyl-3H-2...)Show SMILES CCn1ncc2c1ncc1c(nnc(NCc3ccc(OC)c(Cl)c3)c21)-n1cnc(c1)C(=O)NC Show InChI InChI=1S/C23H22ClN9O2/c1-4-33-21-15(10-29-33)19-14(9-27-21)22(32-11-17(28-12-32)23(34)25-2)31-30-20(19)26-8-13-5-6-18(35-3)16(24)7-13/h5-7,9-12H,4,8H2,1-3H3,(H,25,34)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089814
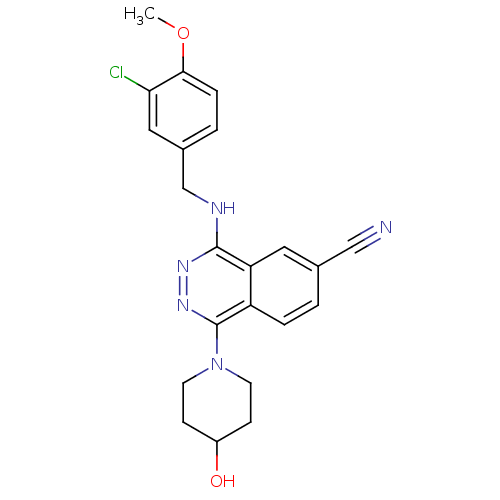
(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxy-pi...)Show SMILES COc1ccc(CNc2nnc(N3CCC(O)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H22ClN5O2/c1-30-20-5-3-15(11-19(20)23)13-25-21-18-10-14(12-24)2-4-17(18)22(27-26-21)28-8-6-16(29)7-9-28/h2-5,10-11,16,29H,6-9,13H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089814

(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxy-pi...)Show SMILES COc1ccc(CNc2nnc(N3CCC(O)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H22ClN5O2/c1-30-20-5-3-15(11-19(20)23)13-25-21-18-10-14(12-24)2-4-17(18)22(27-26-21)28-8-6-16(29)7-9-28/h2-5,10-11,16,29H,6-9,13H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 of human platelets |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14776

(2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...)Show SMILES CCCc1nc(C)c2n1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50123475

(1-[9-(3-Chloro-4-methoxy-benzylamino)-3-ethyl-3H-2...)Show SMILES CCn1ncc2c1ncc1c(nnc(NCc3ccc(OC)c(Cl)c3)c21)N1CCC(O)CC1 Show InChI InChI=1S/C23H26ClN7O2/c1-3-31-22-17(13-27-31)20-16(12-26-22)23(30-8-6-15(32)7-9-30)29-28-21(20)25-11-14-4-5-19(33-2)18(24)10-14/h4-5,10,12-13,15,32H,3,6-9,11H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50098220
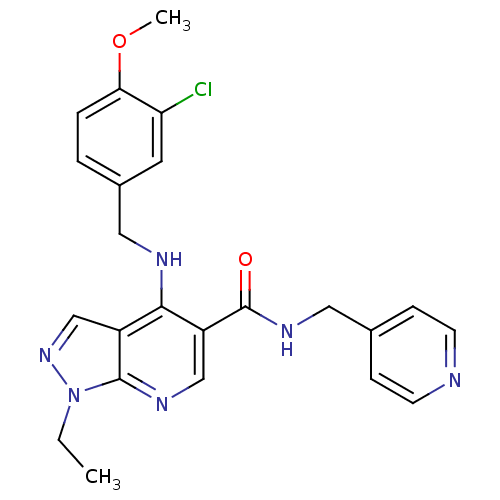
(4-(3-Chloro-4-methoxy-benzylamino)-1-ethyl-1H-pyra...)Show SMILES CCn1ncc2c(NCc3ccc(OC)c(Cl)c3)c(cnc12)C(=O)NCc1ccncc1 Show InChI InChI=1S/C23H23ClN6O2/c1-3-30-22-17(14-29-30)21(26-12-16-4-5-20(32-2)19(24)10-16)18(13-27-22)23(31)28-11-15-6-8-25-9-7-15/h4-10,13-14H,3,11-12H2,1-2H3,(H,26,27)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50098220

(4-(3-Chloro-4-methoxy-benzylamino)-1-ethyl-1H-pyra...)Show SMILES CCn1ncc2c(NCc3ccc(OC)c(Cl)c3)c(cnc12)C(=O)NCc1ccncc1 Show InChI InChI=1S/C23H23ClN6O2/c1-3-30-22-17(14-29-30)21(26-12-16-4-5-20(32-2)19(24)10-16)18(13-27-22)23(31)28-11-15-6-8-25-9-7-15/h4-10,13-14H,3,11-12H2,1-2H3,(H,26,27)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 of human platelets |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50098226
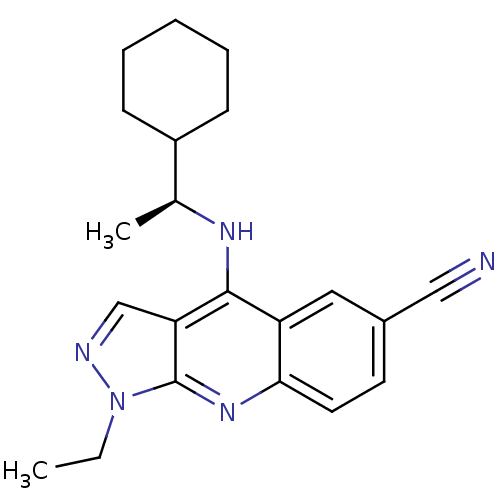
(4-(1-Cyclohexyl-ethylamino)-1-ethyl-1H-pyrazolo[3,...)Show SMILES CCn1ncc2c(N[C@@H](C)C3CCCCC3)c3cc(ccc3nc12)C#N Show InChI InChI=1S/C21H25N5/c1-3-26-21-18(13-23-26)20(24-14(2)16-7-5-4-6-8-16)17-11-15(12-22)9-10-19(17)25-21/h9-11,13-14,16H,3-8H2,1-2H3,(H,24,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 of human platelets |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14777

((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50098225
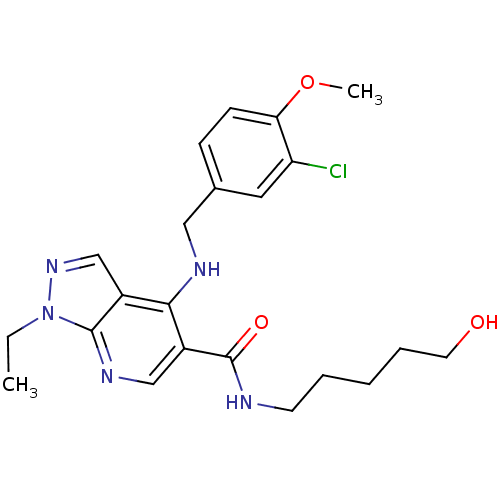
(4-(3-Chloro-4-methoxy-benzylamino)-1-ethyl-1H-pyra...)Show SMILES CCn1ncc2c(NCc3ccc(OC)c(Cl)c3)c(cnc12)C(=O)NCCCCCO Show InChI InChI=1S/C22H28ClN5O3/c1-3-28-21-16(14-27-28)20(25-12-15-7-8-19(31-2)18(23)11-15)17(13-26-21)22(30)24-9-5-4-6-10-29/h7-8,11,13-14,29H,3-6,9-10,12H2,1-2H3,(H,24,30)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 of human platelets |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 of human platelets |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50098219
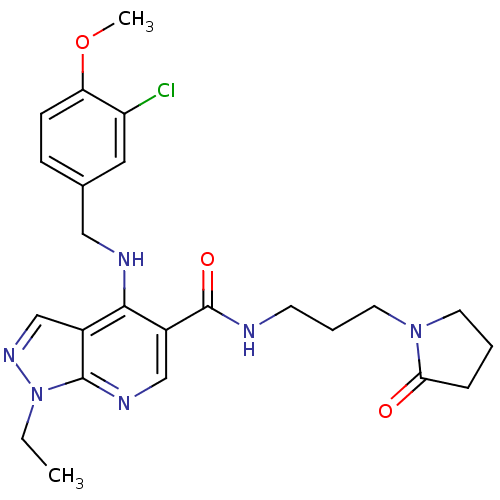
(4-(3-Chloro-4-methoxy-benzylamino)-1-ethyl-1H-pyra...)Show SMILES CCn1ncc2c(NCc3ccc(OC)c(Cl)c3)c(cnc12)C(=O)NCCCN1CCCC1=O Show InChI InChI=1S/C24H29ClN6O3/c1-3-31-23-17(15-29-31)22(27-13-16-7-8-20(34-2)19(25)12-16)18(14-28-23)24(33)26-9-5-11-30-10-4-6-21(30)32/h7-8,12,14-15H,3-6,9-11,13H2,1-2H3,(H,26,33)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 of human platelets |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50098222
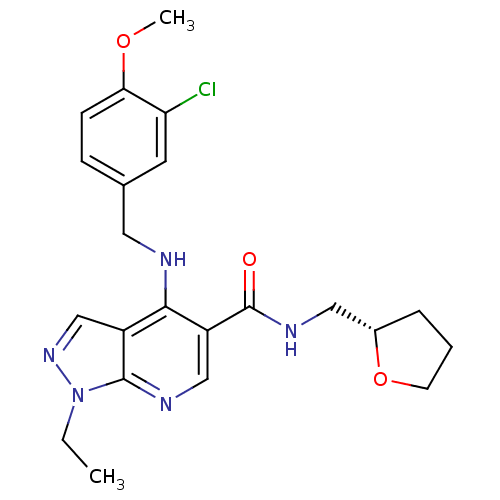
(4-(3-Chloro-4-methoxy-benzylamino)-1-ethyl-1H-pyra...)Show SMILES CCn1ncc2c(NCc3ccc(OC)c(Cl)c3)c(cnc12)C(=O)NC[C@@H]1CCCO1 Show InChI InChI=1S/C22H26ClN5O3/c1-3-28-21-16(13-27-28)20(24-10-14-6-7-19(30-2)18(23)9-14)17(12-25-21)22(29)26-11-15-5-4-8-31-15/h6-7,9,12-13,15H,3-5,8,10-11H2,1-2H3,(H,24,25)(H,26,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 of human platelets |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50098223
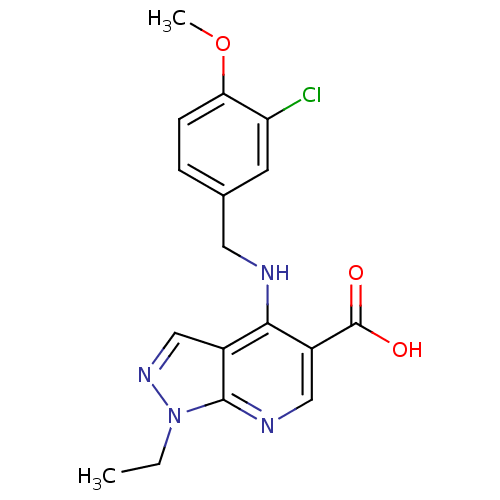
(4-(3-Chloro-4-methoxy-benzylamino)-1-ethyl-1H-pyra...)Show SMILES CCn1ncc2c(NCc3ccc(OC)c(Cl)c3)c(cnc12)C(O)=O Show InChI InChI=1S/C17H17ClN4O3/c1-3-22-16-11(9-21-22)15(12(8-20-16)17(23)24)19-7-10-4-5-14(25-2)13(18)6-10/h4-6,8-9H,3,7H2,1-2H3,(H,19,20)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 of human platelets |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386365
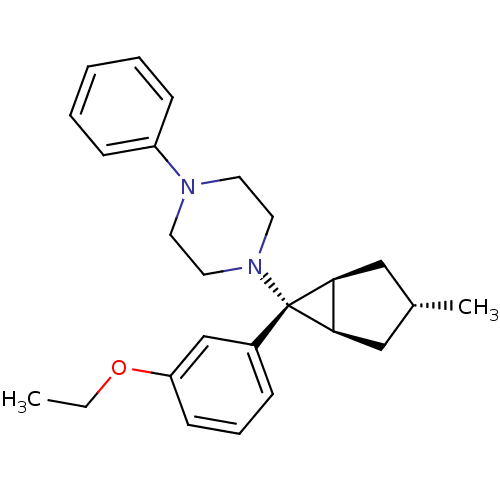
(CHEMBL2046867)Show SMILES CCOc1cccc(c1)[C@]1([C@H]2C[C@H](C)C[C@@H]12)N1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C25H32N2O/c1-3-28-22-11-7-8-20(18-22)25(23-16-19(2)17-24(23)25)27-14-12-26(13-15-27)21-9-5-4-6-10-21/h4-11,18-19,23-24H,3,12-17H2,1-2H3/t19-,23-,24+,25+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386375
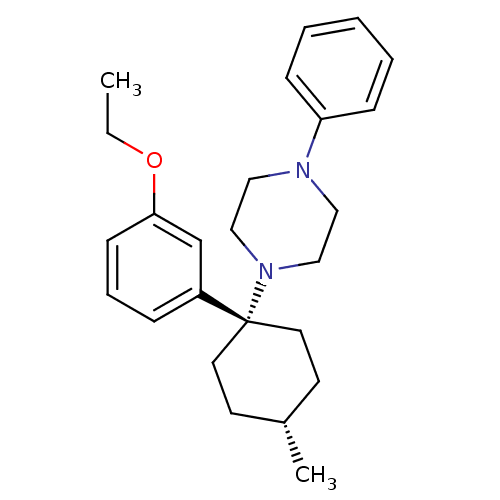
(CHEMBL2046864)Show SMILES CCOc1cccc(c1)[C@]1(CC[C@H](C)CC1)N1CCN(CC1)c1ccccc1 |r,wU:9.17,12.13,(15.93,-8.14,;17.42,-8.55,;18.51,-7.46,;18.12,-5.97,;16.61,-5.56,;16.22,-4.06,;17.33,-2.97,;18.82,-3.39,;19.22,-4.88,;19.93,-2.3,;21.25,-1.54,;21.25,,;19.93,.78,;19.93,2.32,;18.6,,;18.6,-1.54,;21.01,-3.39,;20.6,-4.87,;21.68,-5.96,;23.16,-5.57,;23.57,-4.09,;22.49,-3,;23.92,-6.9,;23.15,-8.22,;23.91,-9.54,;25.44,-9.55,;26.21,-8.22,;25.44,-6.9,)| Show InChI InChI=1S/C25H34N2O/c1-3-28-24-11-7-8-22(20-24)25(14-12-21(2)13-15-25)27-18-16-26(17-19-27)23-9-5-4-6-10-23/h4-11,20-21H,3,12-19H2,1-2H3/t21-,25+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386372

(CHEMBL2046861)Show SMILES C[C@H]1C[C@H]2[C@@H](C1)[C@@]2(N1CCN(CC1)c1ccccc1)c1ccc(O)cc1 |r| Show InChI InChI=1S/C23H28N2O/c1-17-15-21-22(16-17)23(21,18-7-9-20(26)10-8-18)25-13-11-24(12-14-25)19-5-3-2-4-6-19/h2-10,17,21-22,26H,11-16H2,1H3/t17-,21-,22+,23+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50098221
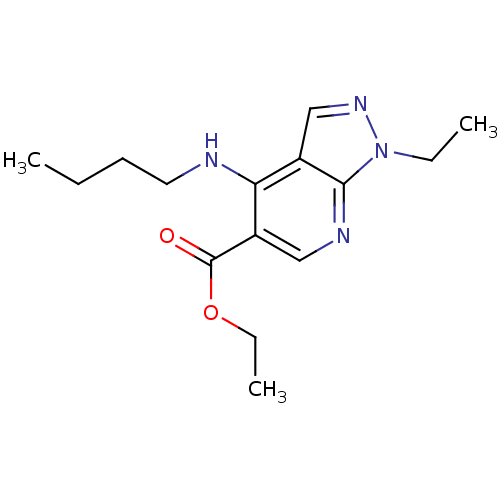
(4-Butylamino-1-ethyl-1H-pyrazolo[3,4-b]pyridine-5-...)Show InChI InChI=1S/C15H22N4O2/c1-4-7-8-16-13-11-10-18-19(5-2)14(11)17-9-12(13)15(20)21-6-3/h9-10H,4-8H2,1-3H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 of human platelets |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386374
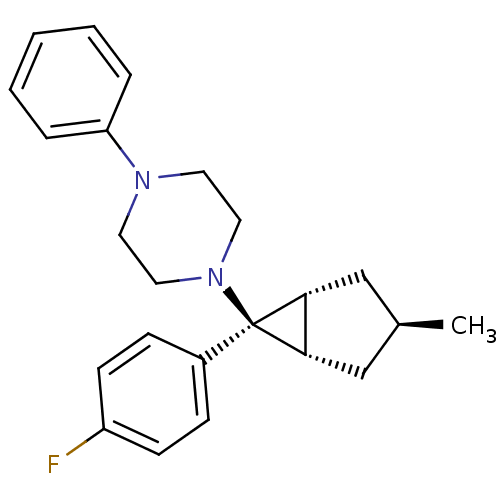
(CHEMBL2046863)Show SMILES C[C@H]1C[C@H]2[C@@H](C1)[C@@]2(N1CCN(CC1)c1ccccc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H27FN2/c1-17-15-21-22(16-17)23(21,18-7-9-19(24)10-8-18)26-13-11-25(12-14-26)20-5-3-2-4-6-20/h2-10,17,21-22H,11-16H2,1H3/t17-,21-,22+,23+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386369
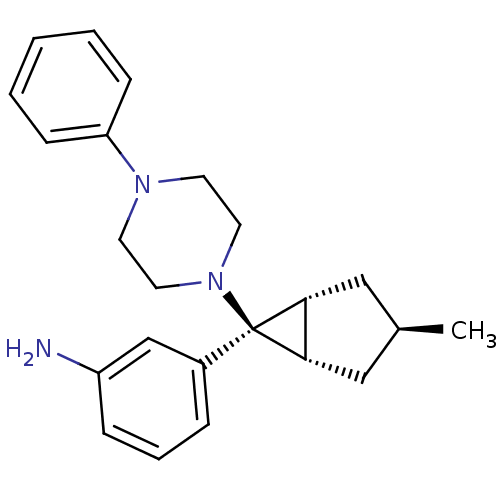
(CHEMBL2046858)Show SMILES C[C@H]1C[C@H]2[C@@H](C1)[C@@]2(N1CCN(CC1)c1ccccc1)c1cccc(N)c1 |r| Show InChI InChI=1S/C23H29N3/c1-17-14-21-22(15-17)23(21,18-6-5-7-19(24)16-18)26-12-10-25(11-13-26)20-8-3-2-4-9-20/h2-9,16-17,21-22H,10-15,24H2,1H3/t17-,21-,22+,23+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386368

(CHEMBL2046870)Show SMILES COc1cccc(c1)[C@]1([C@H]2C[C@H](C)C[C@@H]12)N1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C24H30N2O/c1-18-15-22-23(16-18)24(22,19-7-6-10-21(17-19)27-2)26-13-11-25(12-14-26)20-8-4-3-5-9-20/h3-10,17-18,22-23H,11-16H2,1-2H3/t18-,22-,23+,24+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386371
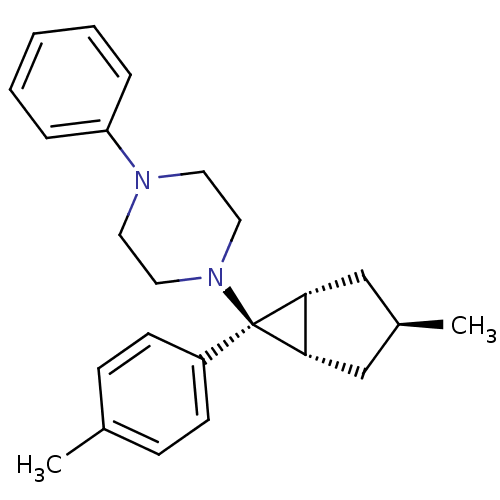
(CHEMBL2046860)Show SMILES C[C@H]1C[C@H]2[C@@H](C1)[C@@]2(N1CCN(CC1)c1ccccc1)c1ccc(C)cc1 |r| Show InChI InChI=1S/C24H30N2/c1-18-8-10-20(11-9-18)24(22-16-19(2)17-23(22)24)26-14-12-25(13-15-26)21-6-4-3-5-7-21/h3-11,19,22-23H,12-17H2,1-2H3/t19-,22-,23+,24+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386373
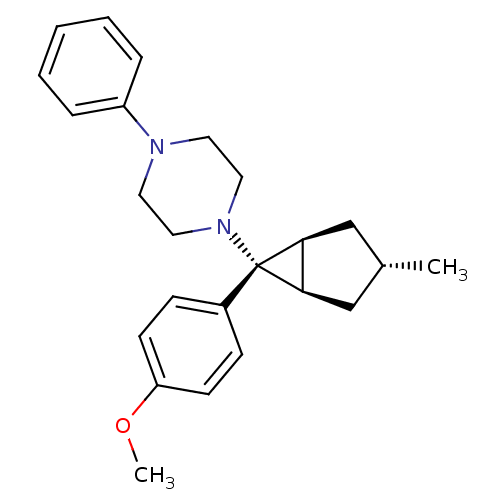
(CHEMBL2046862)Show SMILES COc1ccc(cc1)[C@]1([C@H]2C[C@H](C)C[C@@H]12)N1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C24H30N2O/c1-18-16-22-23(17-18)24(22,19-8-10-21(27-2)11-9-19)26-14-12-25(13-15-26)20-6-4-3-5-7-20/h3-11,18,22-23H,12-17H2,1-2H3/t18-,22-,23+,24+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386367
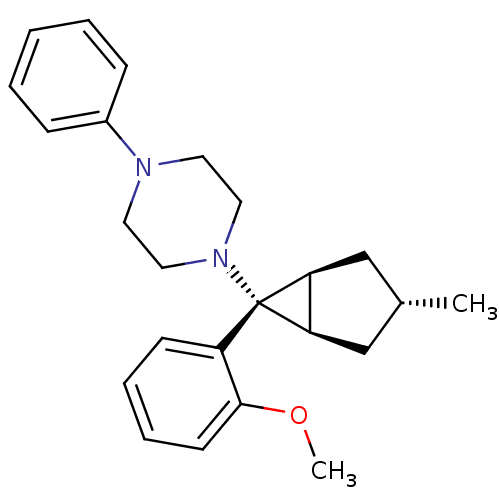
(CHEMBL2046869)Show SMILES COc1ccccc1[C@]1([C@H]2C[C@H](C)C[C@@H]12)N1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C24H30N2O/c1-18-16-21-22(17-18)24(21,20-10-6-7-11-23(20)27-2)26-14-12-25(13-15-26)19-8-4-3-5-9-19/h3-11,18,21-22H,12-17H2,1-2H3/t18-,21-,22+,24+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
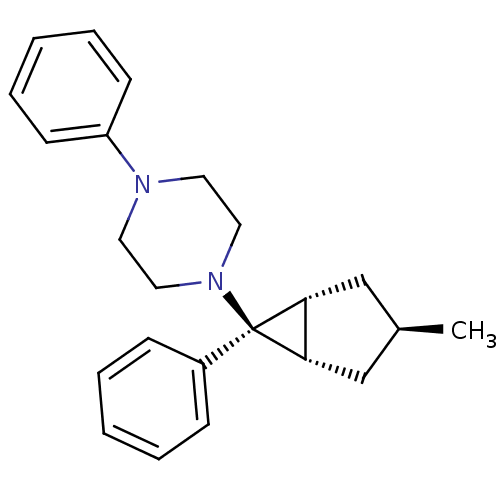
(Homo sapiens (Human)) | BDBM50386366
(CHEMBL2046868)Show SMILES C[C@H]1C[C@H]2[C@@H](C1)[C@@]2(N1CCN(CC1)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C23H28N2/c1-18-16-21-22(17-18)23(21,19-8-4-2-5-9-19)25-14-12-24(13-15-25)20-10-6-3-7-11-20/h2-11,18,21-22H,12-17H2,1H3/t18-,21-,22+,23+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50386370

(CHEMBL2046859)Show SMILES C[C@H]1C[C@H]2[C@@H](C1)[C@@]2(N1CCN(CC1)c1ccccc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C23H27ClN2/c1-17-14-21-22(15-17)23(21,18-6-5-7-19(24)16-18)26-12-10-25(11-13-26)20-8-3-2-4-9-20/h2-9,16-17,21-22H,10-15H2,1H3/t17-,21-,22+,23+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting |
ACS Med Chem Lett 3: 222-226 (2012)
Article DOI: 10.1021/ml200265m
BindingDB Entry DOI: 10.7270/Q2ZP475V |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50098220

(4-(3-Chloro-4-methoxy-benzylamino)-1-ethyl-1H-pyra...)Show SMILES CCn1ncc2c(NCc3ccc(OC)c(Cl)c3)c(cnc12)C(=O)NCc1ccncc1 Show InChI InChI=1S/C23H23ClN6O2/c1-3-30-22-17(14-29-30)21(26-12-16-4-5-20(32-2)19(24)10-16)18(13-27-22)23(31)28-11-15-6-8-25-9-7-15/h4-10,13-14H,3,11-12H2,1-2H3,(H,26,27)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Relaxant effect on rabbit corpus cavernosal tissue strips |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Relaxant effect on rabbit corpus cavernosal tissue strips |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data