

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
C.N.R.S. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Butyrylcholinesterase (BChE) in human serum | Bioorg Med Chem Lett 13: 2389-91 (2003) BindingDB Entry DOI: 10.7270/Q22R3R2Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM19126 ((1R,3R,6R,7S,8R,10S,11S,14R,15R,20S)-18-[(2S)-buta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM19125 ((1R,3R,6R,7S,8R,10S,11S,14R,15R,20S)-7-[(1S)-1-(di...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19110 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-[(2S)-butan-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19126 ((1R,3R,6R,7S,8R,10S,11S,14R,15R,20S)-18-[(2S)-buta...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
C.N.R.S. Curated by ChEMBL | Assay Description In vitro inhibitory activity against acetylcholinesterase (AChE) in Electrophorus electricus | Bioorg Med Chem Lett 13: 2389-91 (2003) BindingDB Entry DOI: 10.7270/Q22R3R2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19125 ((1R,3R,6R,7S,8R,10S,11S,14R,15R,20S)-7-[(1S)-1-(di...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19111 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-(butan-2-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19129 ((3R,6R,7S,8R,10S,11R,14R,15R,20S)-18-[(2S)-butan-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19101 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of BuChE activity was determined by the spectroscopic method of Ellman using butyrylthiocholine iodide as substrate, in 96-well microtiter... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19112 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19104 ((2S)-N-[(1R,6S,7S,8R,11R,12S,14R,15S,16R)-15-[(1S)...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19122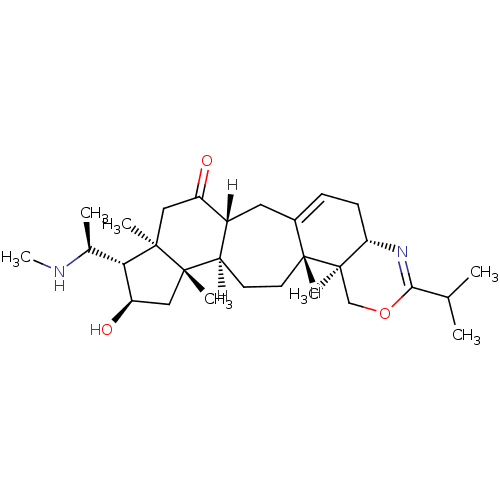 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-8-hydroxy-6,10,1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19118 ((1S,6R,7S,8R,10S,11S,14R,15S,20S)-7-[(1S)-1-(dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19120 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19121 (N-[(6S,7S,8R,11S,12S,14R,15S,16R)-14-hydroxy-7-(hy...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19105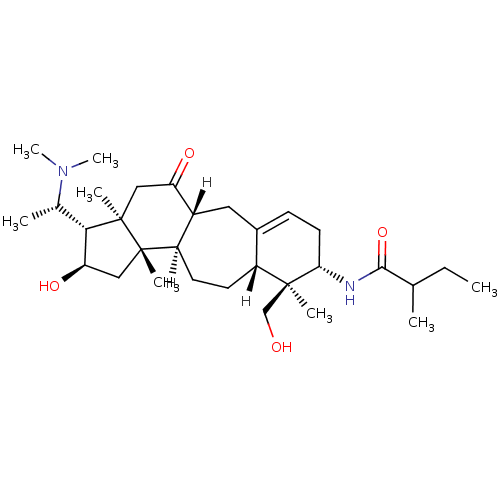 (N-[(1R,6S,7S,8R,11R,12S,14R,15S,16R)-15-[(1S)-1-(d...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19109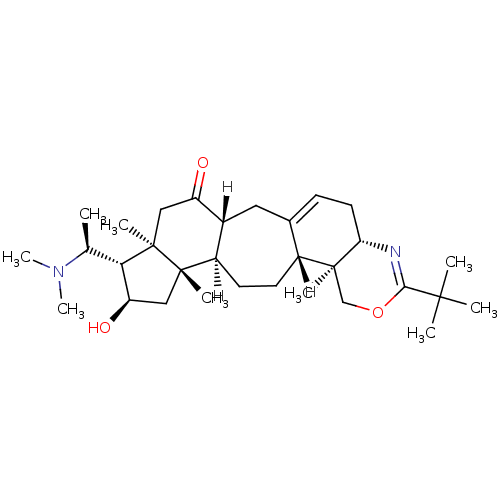 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-tert-butyl-7-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 225 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM19110 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-[(2S)-butan-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM19110 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-[(2S)-butan-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19123 (N-[(1R,3R,6S,7R,8R,11S,12S,14R,15S,16R)-7-(aminome...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM19101 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 299 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM19109 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-tert-butyl-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 351 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19128 ((2S)-N-[(1R,6S,7R,8R,11R,12S,14R,15S,16R)-7-(amino...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 368 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM19109 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-tert-butyl-7-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM19114 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-cyclohexyl-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 375 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of BuChE activity was determined by the spectroscopic method of Ellman using butyrylthiocholine iodide as substrate, in 96-well microtiter... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM19115 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-benzyl-7-[(1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of BuChE activity was determined by the spectroscopic method of Ellman using butyrylthiocholine iodide as substrate, in 96-well microtiter... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19119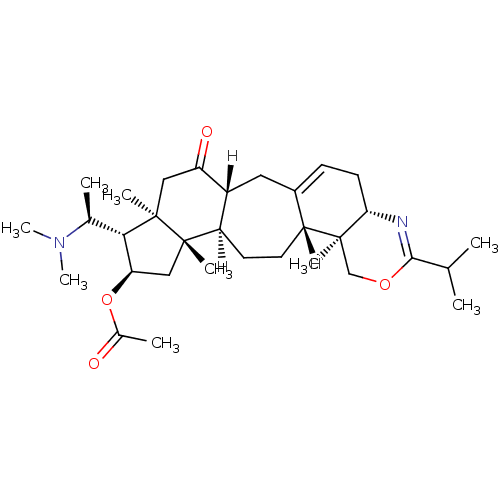 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM19101 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 385 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19114 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-cyclohexyl-7-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 446 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM19129 ((3R,6R,7S,8R,10S,11R,14R,15R,20S)-18-[(2S)-butan-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 447 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM19122 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-8-hydroxy-6,10,1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 485 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM19122 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-8-hydroxy-6,10,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 517 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19108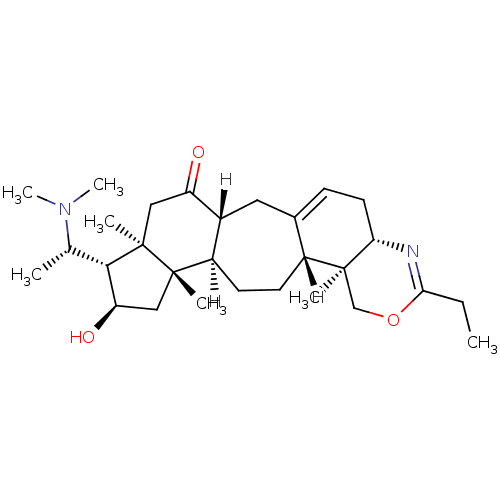 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 607 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19107 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM19120 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 724 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM19101 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 756 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19127 ((2S)-N-[(1R,6S,7S,8R,11R,12S,14R,15S,16R)-7-[(1E)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19116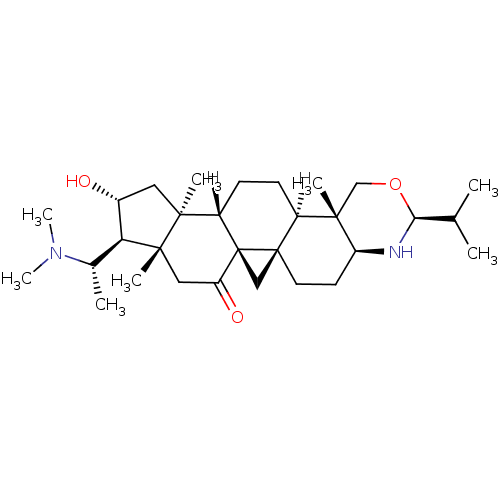 ((1R,3R,6R,7S,8R,10S,11S,14R,15S,18S,20S)-7-[(1S)-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM19120 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 839 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM19101 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS | Assay Description Inhibition of BuChE activity was determined by the spectroscopic method of Ellman using butyrylthiocholine iodide as substrate, in 96-well microtiter... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM19109 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-tert-butyl-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM19124 ((2S)-N-[(1R,3R,6S,7R,8R,11S,12S,14R,15S,16R)-7-(am...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM19110 ((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-[(2S)-butan-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... | J Med Chem 50: 5311-23 (2007) Article DOI: 10.1021/jm070536w BindingDB Entry DOI: 10.7270/Q22B8W82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |