

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by Pfizer mobility shift assay | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018836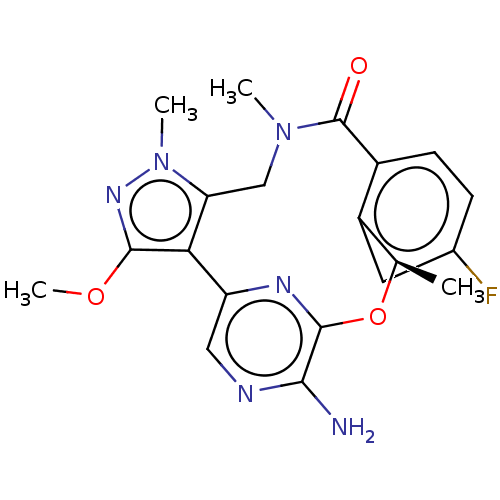 (CHEMBL3286826) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50018830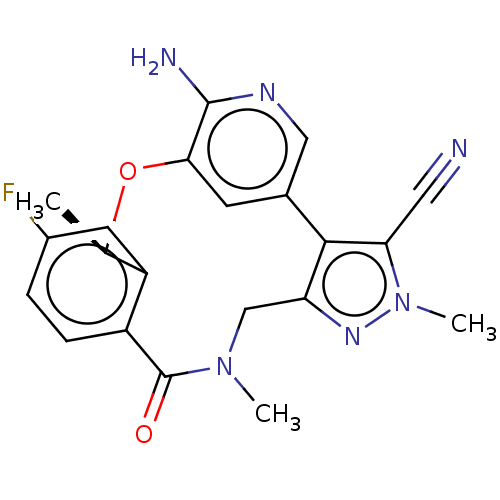 (CHEMBL3286830 | US10543199, Compound PF-06463922 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by off-chip mobility shift assay | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50369022 (CHEMBL1788197) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against cloned mammalian 5-hydroxytryptamine 1A receptor expressed in CHO cells, by using [3H]8-OH-DPAT as radioligand. | J Med Chem 38: 2217-30 (1995) BindingDB Entry DOI: 10.7270/Q28G8MBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498218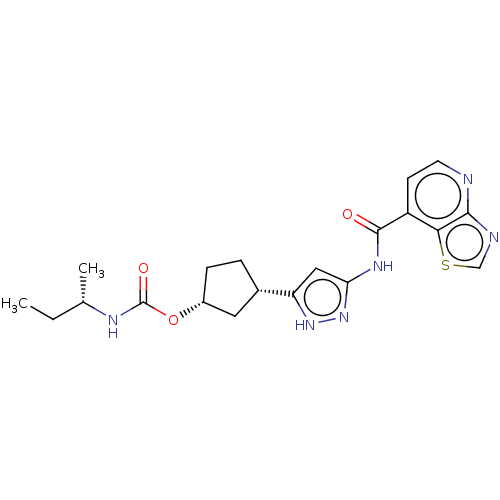 (US11014911, Example 43 | US11718603, Example 43) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50035336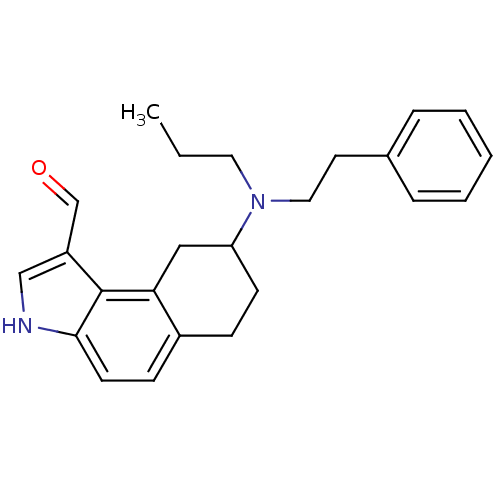 (8-(Phenethyl-propyl-amino)-6,7,8,9-tetrahydro-3H-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against cloned mammalian 5-hydroxytryptamine 1A receptor expressed in CHO cells, by using [3H]8-OH-DPAT as radioligand. | J Med Chem 38: 2217-30 (1995) BindingDB Entry DOI: 10.7270/Q28G8MBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498218 (US11014911, Example 43 | US11718603, Example 43) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370133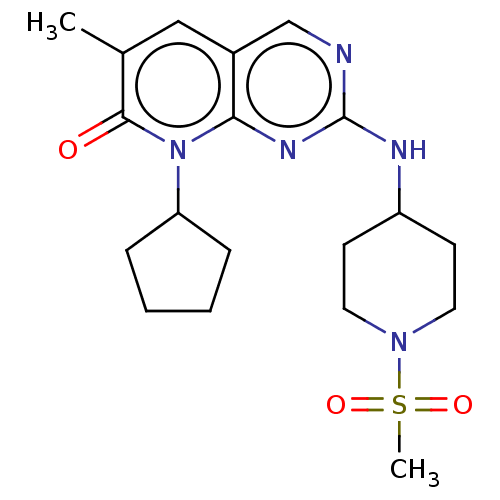 (US10233188, Example 22 | US10800783, Example 22 | ...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498217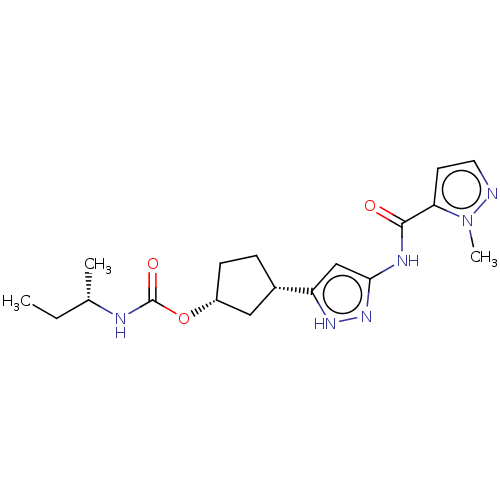 (US11014911, Example 42 | US11718603, Example 42) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498217 (US11014911, Example 42 | US11718603, Example 42) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498324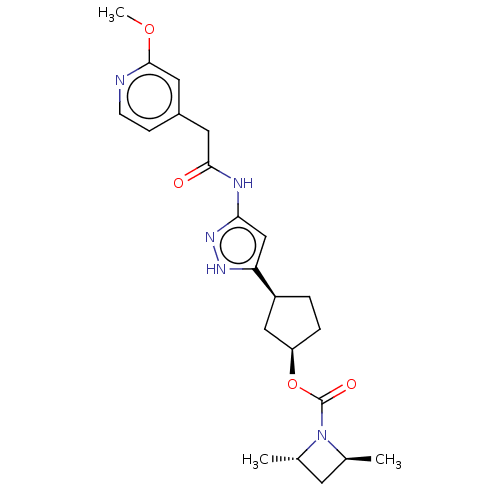 (US11014911, Example 149 | US11718603, Example 149) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498324 (US11014911, Example 149 | US11718603, Example 149) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370115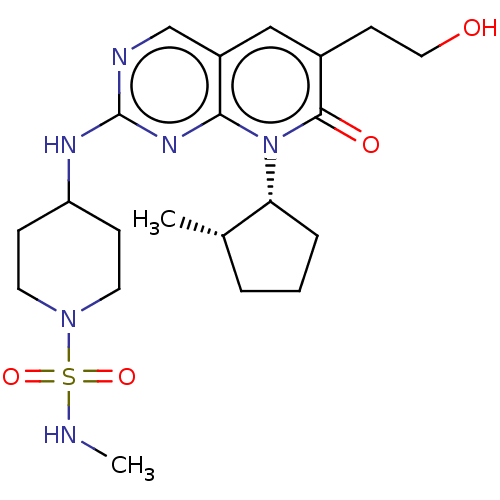 (4-({6-(2-hydroxyethyl)-8-[(1R,2S)-2-methylcyclopen...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The mobility shift assay electrophoretically separates the fluorescently labeled peptides (substrate and phosphorylated product) following the kinase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370149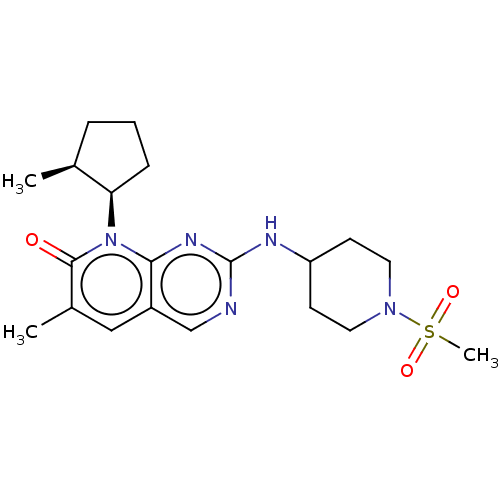 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The mobility shift assay electrophoretically separates the fluorescently labeled peptides (substrate and phosphorylated product) following the kinase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM370149 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 (Homo sapiens (Human)-Mus musculus (mouse)) | BDBM370149 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370115 (4-({6-(2-hydroxyethyl)-8-[(1R,2S)-2-methylcyclopen...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370149 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 (Homo sapiens (Human)-Mus musculus (mouse)) | BDBM370149 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370115 (4-({6-(2-hydroxyethyl)-8-[(1R,2S)-2-methylcyclopen...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370149 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM467013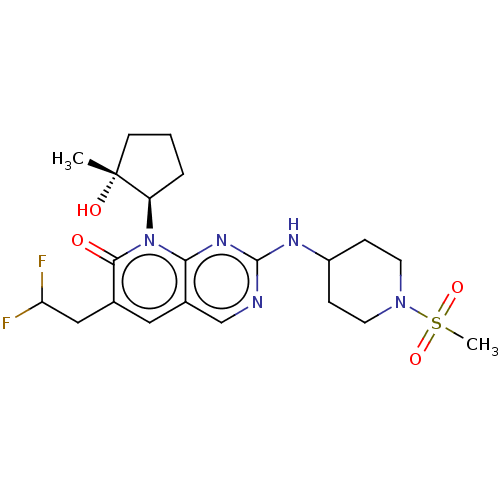 ((-)-6-(2,2-difluoroethyl)-8-[(1R*,2R*)-2-hydroxy-2...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498778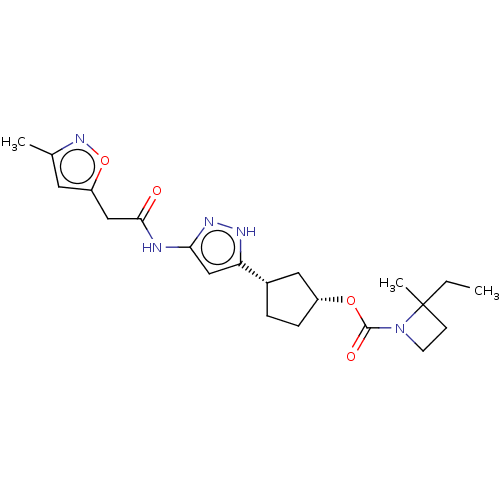 ((1R,3S)-3-(3-{[(3-methyl-1,2- oxazol-5-yl)acetyl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498321 (US11014911, Example 146 | US11718603, Example 146) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498778 ((1R,3S)-3-(3-{[(3-methyl-1,2- oxazol-5-yl)acetyl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50035342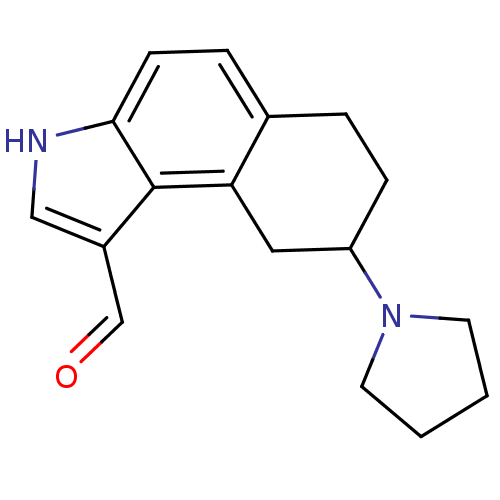 (8-Pyrrolidin-1-yl-6,7,8,9-tetrahydro-3H-benzo[e]in...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against cloned mammalian 5-hydroxytryptamine 1A receptor expressed in CHO cells, by using [3H]8-OH-DPAT as radioligand. | J Med Chem 38: 2217-30 (1995) BindingDB Entry DOI: 10.7270/Q28G8MBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018830 (CHEMBL3286830 | US10543199, Compound PF-06463922 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018837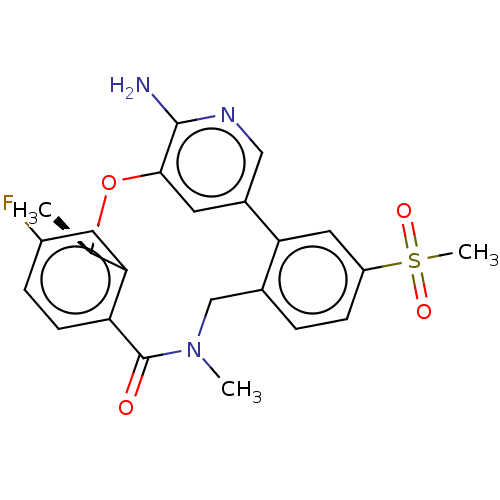 (CHEMBL3286827) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498321 (US11014911, Example 146 | US11718603, Example 146) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498778 ((1R,3S)-3-(3-{[(3-methyl-1,2- oxazol-5-yl)acetyl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018836 (CHEMBL3286826) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370300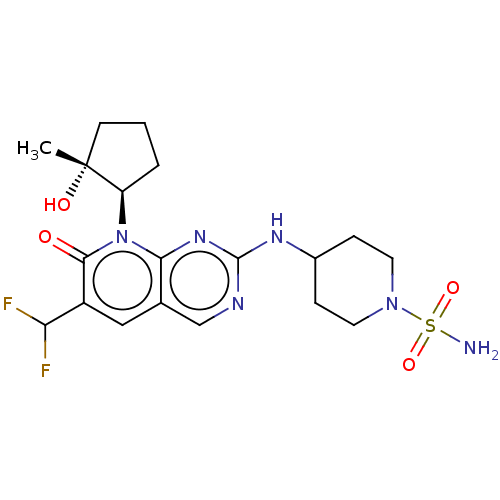 (BDBM467195 | US10233188, Example 187) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The mobility shift assay electrophoretically separates the fluorescently labeled peptides (substrate and phosphorylated product) following the kinase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498316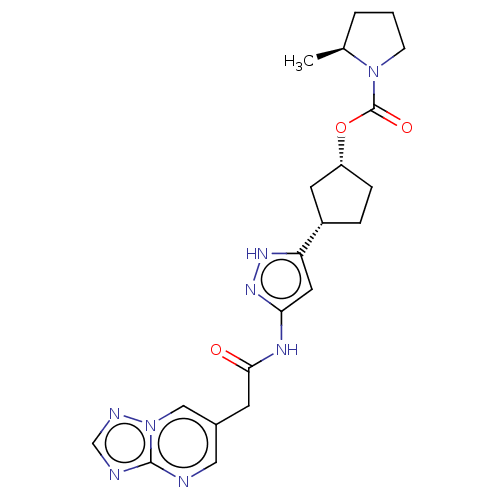 (US11014911, Example 141 | US11718603, Example 141) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370197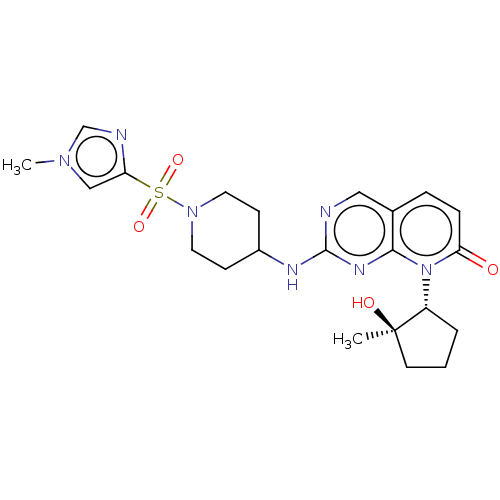 (US10233188, Example 84 | US10800783, Example 84 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The mobility shift assay electrophoretically separates the fluorescently labeled peptides (substrate and phosphorylated product) following the kinase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370205 (US10233188, Example 92 | US10800783, Example 92 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The mobility shift assay electrophoretically separates the fluorescently labeled peptides (substrate and phosphorylated product) following the kinase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM370121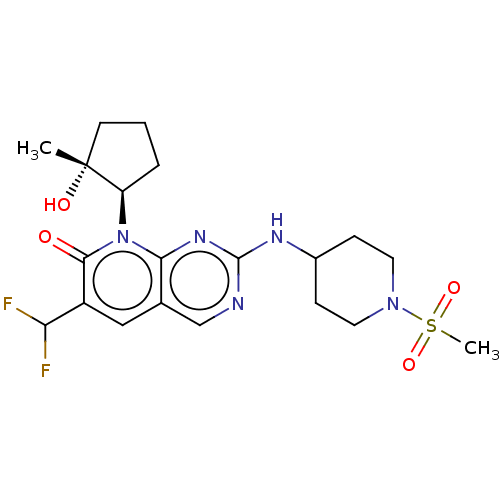 (6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370152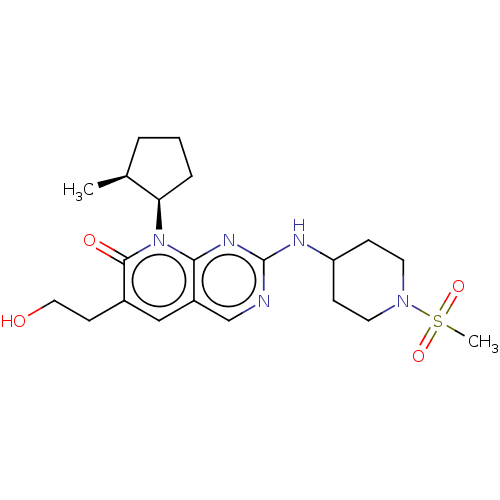 (US10233188, Example 40 | US10800783, Example 40 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The mobility shift assay electrophoretically separates the fluorescently labeled peptides (substrate and phosphorylated product) following the kinase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM370162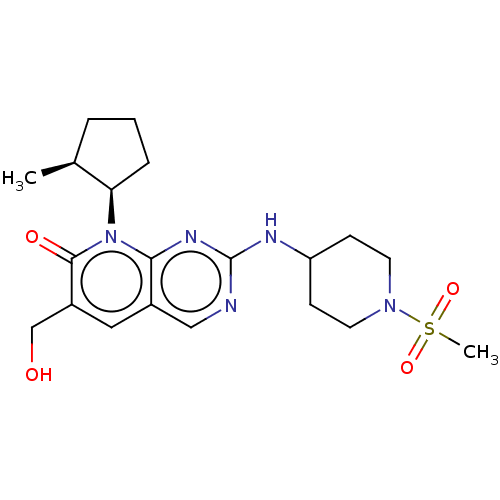 (US10233188, Example 50 | US10800783, Example 50 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370205 (US10233188, Example 92 | US10800783, Example 92 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 (Homo sapiens (Human)-Mus musculus (mouse)) | BDBM370162 (US10233188, Example 50 | US10800783, Example 50 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498778 ((1R,3S)-3-(3-{[(3-methyl-1,2- oxazol-5-yl)acetyl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498316 (US11014911, Example 141 | US11718603, Example 141) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498295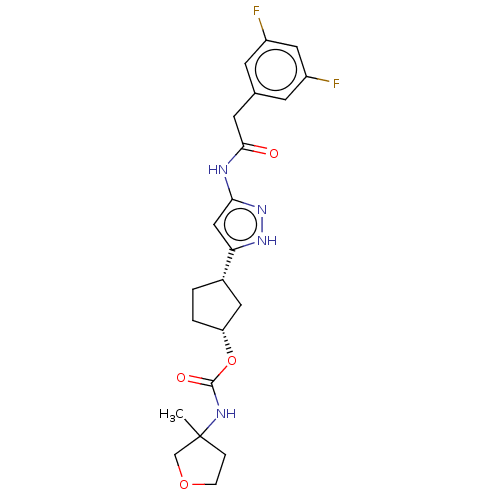 (US11014911, Example 120 | US11014911, Example 121 ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370197 (US10233188, Example 84 | US10800783, Example 84 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370205 (US10233188, Example 92 | US10800783, Example 92 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370300 (BDBM467195 | US10233188, Example 187) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 (Homo sapiens (Human)-Mus musculus (mouse)) | BDBM370121 (6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370152 (US10233188, Example 40 | US10800783, Example 40 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4955 total ) | Next | Last >> |