

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Tested for inhibitor binding of wild-type HIV PR | J Med Chem 47: 2030-6 (2004) Article DOI: 10.1021/jm031105q BindingDB Entry DOI: 10.7270/Q2CN74PV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50581791 (CHEMBL5073221) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV-1 protease assessed as initial inhibition constant by spectrophotometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02177 BindingDB Entry DOI: 10.7270/Q208695M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50143837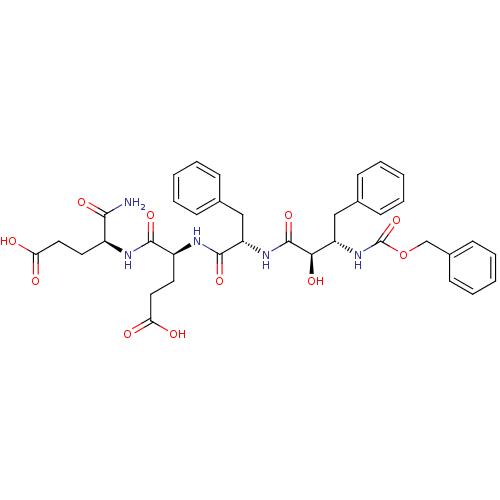 ((S)-4-[(S)-2-((2R,3S)-3-Benzyloxycarbonylamino-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Tested for inhibitor binding of Val82Ala mutant of HIV PR | J Med Chem 47: 2030-6 (2004) Article DOI: 10.1021/jm031105q BindingDB Entry DOI: 10.7270/Q2CN74PV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Tested for inhibitor binding of D25N/V82A mutant of HIV PR | J Med Chem 47: 2030-6 (2004) Article DOI: 10.1021/jm031105q BindingDB Entry DOI: 10.7270/Q2CN74PV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50143837 ((S)-4-[(S)-2-((2R,3S)-3-Benzyloxycarbonylamino-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Tested for inhibitor binding of wild-type HIV PR | J Med Chem 47: 2030-6 (2004) Article DOI: 10.1021/jm031105q BindingDB Entry DOI: 10.7270/Q2CN74PV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50581791 (CHEMBL5073221) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human cathepsin E assessed as inhibition constant using ACC-GKPILFFRILK-(DNP)-(dR)-NH2 as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02177 BindingDB Entry DOI: 10.7270/Q208695M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50581791 (CHEMBL5073221) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human cathepsin D assessed as inhibition constant using ACC-GKPILFFRILK-(DNP)-(dR)-NH2 as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02177 BindingDB Entry DOI: 10.7270/Q208695M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50581791 (CHEMBL5073221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human renin assessed as inhibition constant using H-R-E(EDANS)-1HPFHLVIHT-K(DABCYL)-R-OH as susbtrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02177 BindingDB Entry DOI: 10.7270/Q208695M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||