

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50007692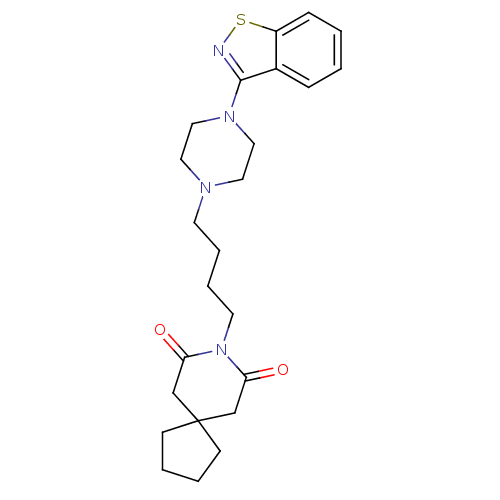 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at 5-hydroxytryptamine 2 receptor in rat cortical by [3H]spiroperidol displacement. | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the absence of Zn | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at 5-hydroxytryptamine 2 receptor in rat cortical by [3H]spiroperidol displacement. | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225539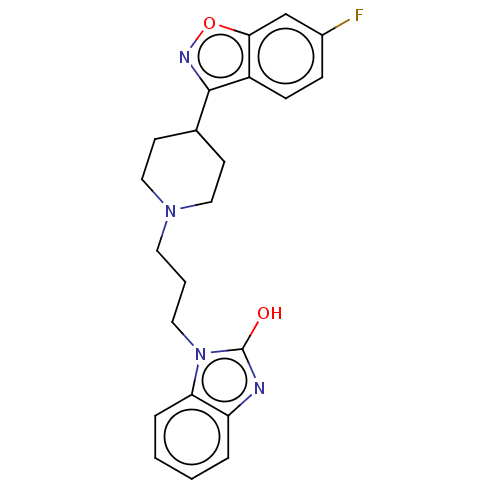 (CHEMBL164940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048608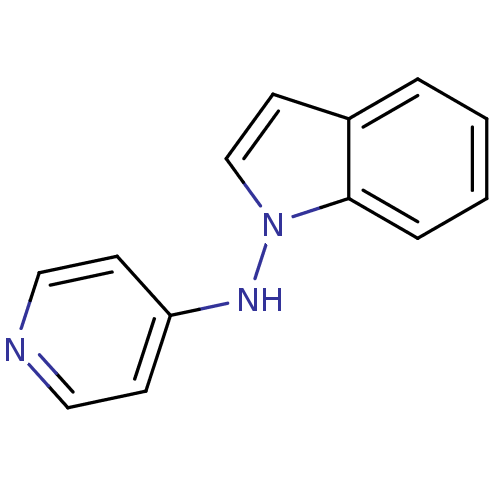 (CHEMBL154488 | Indol-1-yl-pyridin-4-yl-amine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10972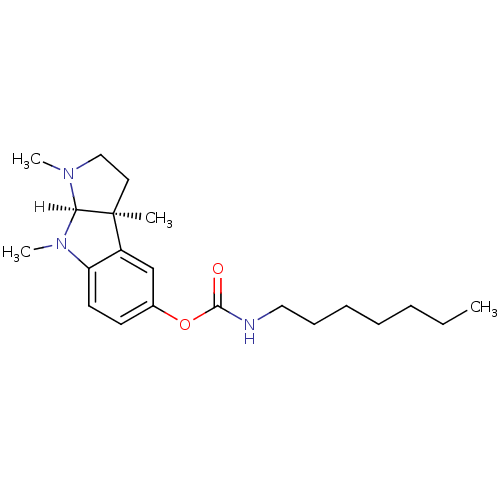 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50007498 (8-[3-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-pr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at 5-hydroxytryptamine 2 receptor in rat cortical by [3H]spiroperidol displacement. | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50007483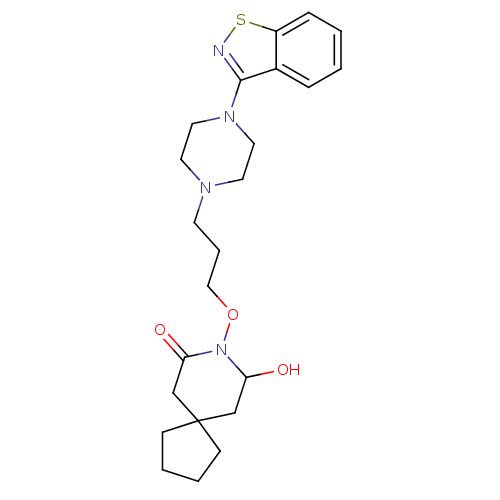 (8-[3-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-pr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at 5-hydroxytryptamine 2 receptor in rat cortical by [3H]spiroperidol displacement. | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9349 ((2Z)-but-2-enedioic acid; 9-amino-6-chloro-1,2,3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hoechst-Roussel Pharmaceuticals, Inc. | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. | J Med Chem 32: 1805-13 (1989) Article DOI: 10.1021/jm00128a024 BindingDB Entry DOI: 10.7270/Q24Q7S6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225526 (CHEMBL350575) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225528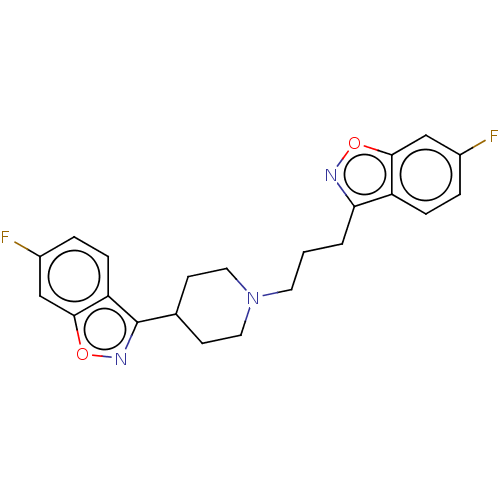 (CHEMBL168104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007692 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50007481 (8-[3-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-pr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at 5-hydroxytryptamine 2 receptor in rat cortical by [3H]spiroperidol displacement. | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at dopamine receptor D2 labeled with [3H]spiroperidol radioligand in striatum tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225534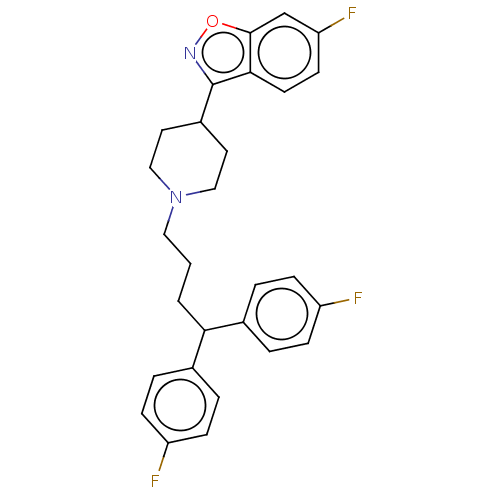 (CHEMBL351954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007692 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at dopamine receptor D2 labeled with [3H]spiroperidol radioligand in striatum tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048589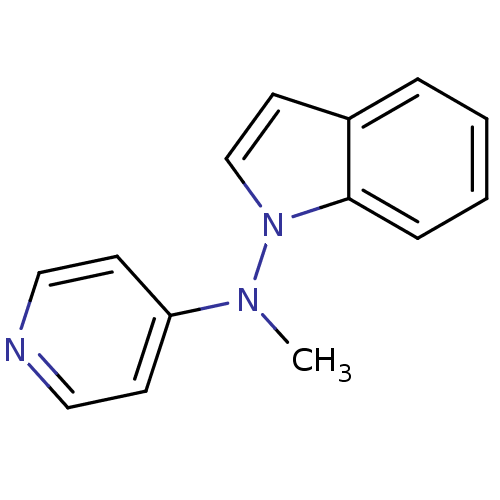 (CHEMBL152842 | Indol-1-yl-methyl-pyridin-4-yl-amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225543 (CHEMBL165301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007505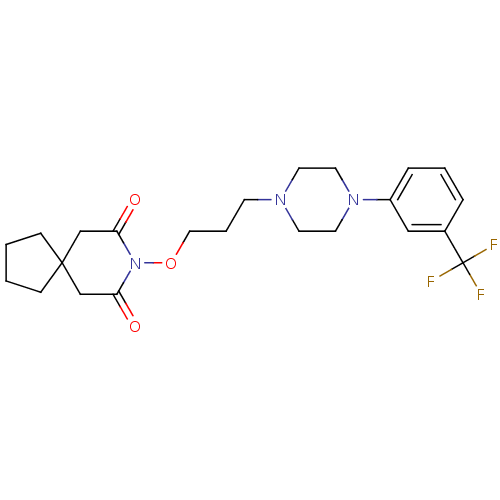 (8-{3-[4-(3-Trifluoromethyl-phenyl)-piperazin-1-yl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007498 (8-[3-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-pr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at dopamine receptor D2 labeled with [3H]spiroperidol radioligand in striatum tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at 5-hydroxytryptamine 2 receptor in rat cortical by [3H]spiroperidol displacement. | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007494 (8-{3-[4-(3-Chloro-phenyl)-piperazin-1-yl]-propoxy}...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225524 (CHEMBL165775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225542 (CHEMBL166095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007496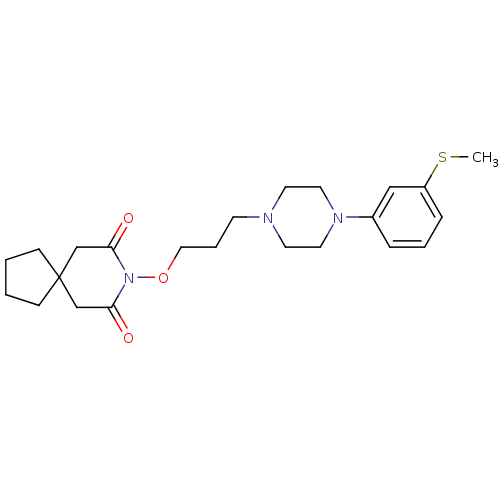 (8-{3-[4-(3-Methylsulfanyl-phenyl)-piperazin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007495 (9-Hydroxy-8-{3-[4-(2-methoxy-phenyl)-piperazin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50231956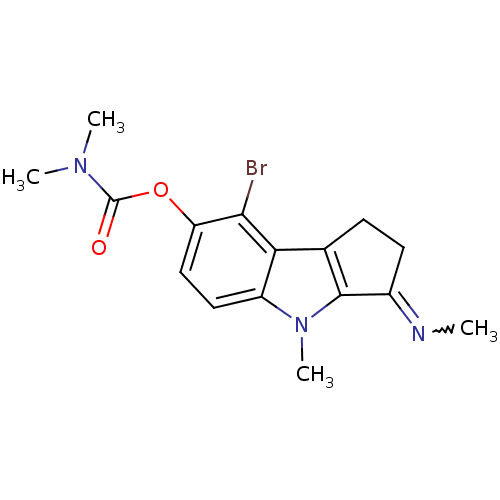 ((Z)-8-bromo-4-methyl-3-(methylimino)-1,2,3,4-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225545 (CHEMBL165857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225538 (CHEMBL166311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007492 (8-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-propoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225535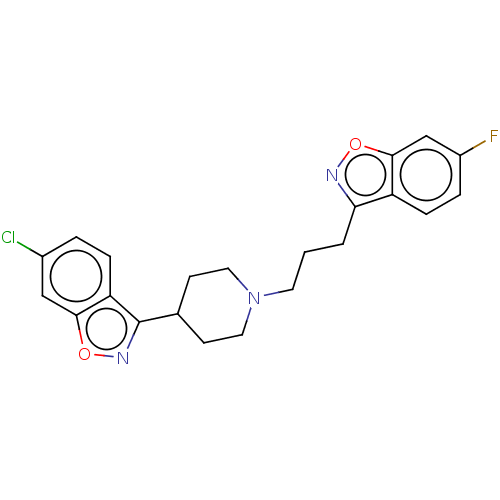 (CHEMBL349840) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50004303 (CHEMBL344115 | Phenyl-piperidin-4-yl-methanone) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007483 (8-[3-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-pr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225522 (CHEMBL165576) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007484 (8-[3-(4-m-Tolyl-piperazin-1-yl)-propoxy]-8-aza-spi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50288976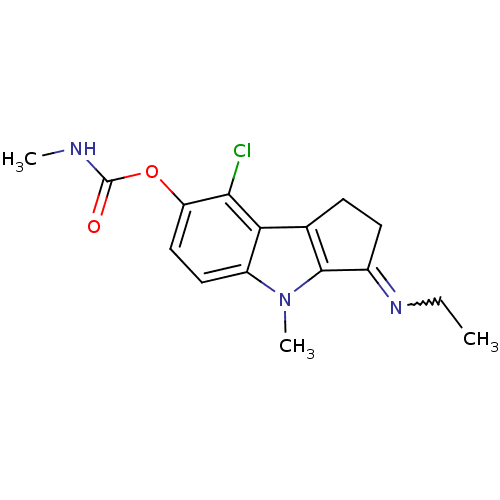 (CHEMBL155350 | Methyl-carbamic acid 8-chloro-3-[(Z...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007480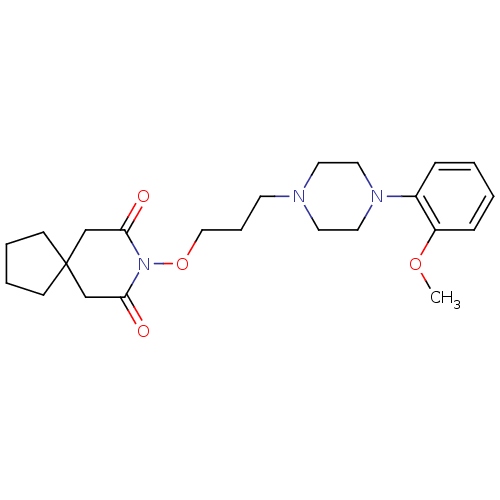 (8-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-propoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007481 (8-[3-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-pr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 1A receptor labeled with [3H]8-OH-DPAT radioligand in hippocampus tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048600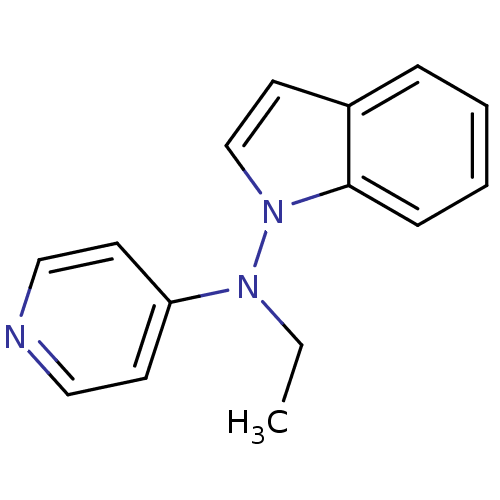 (CHEMBL154541 | Ethyl-indol-1-yl-pyridin-4-yl-amine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at 5-hydroxytryptamine 2 receptor in rat cortical by [3H]spiroperidol displacement. | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225531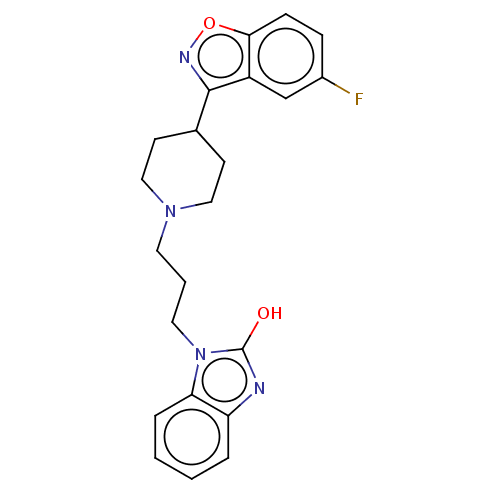 (CHEMBL349632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225525 (CHEMBL164973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiroperidol in rat striatal tissue | J Med Chem 28: 761-9 (1985) BindingDB Entry DOI: 10.7270/Q2F47RCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [3H]idazoxan binding to Alpha-2 adrenergic receptor in rat cortex, in the presence of GPP | J Med Chem 39: 570-81 (1996) Article DOI: 10.1021/jm9506433 BindingDB Entry DOI: 10.7270/Q2BR8R7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007481 (8-[3-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was measured for affinity at dopamine receptor D2 labeled with [3H]spiroperidol radioligand in striatum tissue | J Med Chem 34: 1068-72 (1991) BindingDB Entry DOI: 10.7270/Q2D21Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50288977 (CHEMBL160836 | Methyl-carbamic acid 8-bromo-3-[(Z)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 492 total ) | Next | Last >> |