 Found 261 hits with Last Name = 'hughes' and Initial = 'pf'
Found 261 hits with Last Name = 'hughes' and Initial = 'pf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50450704
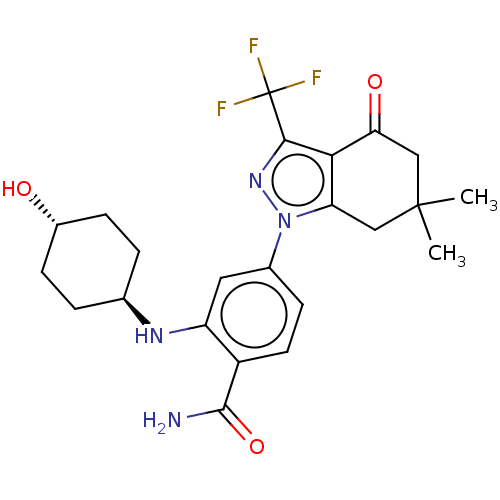
(CHEMBL560895 | SNX-2112)Show SMILES CC1(C)Cc2c(c(nn2-c2ccc(C(N)=O)c(N[C@H]3CC[C@H](O)CC3)c2)C(F)(F)F)C(=O)C1 |r,wU:18.18,wD:21.22,(-2.35,-2,;-2.38,-.77,;-3.43,-.12,;-1.03,-1.55,;.3,-.77,;.3,.77,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.68,;3.35,-7.18,;4.53,-7.52,;2.46,-8.03,;4.09,-4.62,;5.57,-5.04,;6.68,-3.97,;6.3,-2.47,;7.41,-1.4,;8.89,-1.82,;9.77,-.96,;9.27,-3.32,;8.16,-4.39,;3.72,-3.12,;2.24,2.7,;3.44,2.95,;1.41,3.61,;2.62,3.87,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,)| Show InChI InChI=1S/C23H27F3N4O3/c1-22(2)10-17-19(18(32)11-22)20(23(24,25)26)29-30(17)13-5-8-15(21(27)33)16(9-13)28-12-3-6-14(31)7-4-12/h5,8-9,12,14,28,31H,3-4,6-7,10-11H2,1-2H3,(H2,27,33)/t12-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human A375 cells assessed as pS6 degradation after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50128787
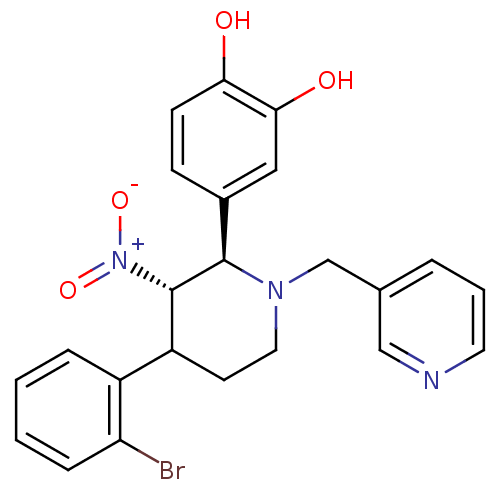
(4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...)Show SMILES Oc1ccc(cc1O)[C@@H]1[C@H](C(CCN1Cc1cccnc1)c1ccccc1Br)[N+]([O-])=O Show InChI InChI=1S/C23H22BrN3O4/c24-19-6-2-1-5-17(19)18-9-11-26(14-15-4-3-10-25-13-15)22(23(18)27(30)31)16-7-8-20(28)21(29)12-16/h1-8,10,12-13,18,22-23,28-29H,9,11,14H2/t18?,22-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras |
J Med Chem 46: 2467-73 (2003)
Article DOI: 10.1021/jm020522k
BindingDB Entry DOI: 10.7270/Q2RN3775 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50128787

(4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...)Show SMILES Oc1ccc(cc1O)[C@@H]1[C@H](C(CCN1Cc1cccnc1)c1ccccc1Br)[N+]([O-])=O Show InChI InChI=1S/C23H22BrN3O4/c24-19-6-2-1-5-17(19)18-9-11-26(14-15-4-3-10-25-13-15)22(23(18)27(30)31)16-7-8-20(28)21(29)12-16/h1-8,10,12-13,18,22-23,28-29H,9,11,14H2/t18?,22-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras |
J Med Chem 46: 2467-73 (2003)
Article DOI: 10.1021/jm020522k
BindingDB Entry DOI: 10.7270/Q2RN3775 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50450704

(CHEMBL560895 | SNX-2112)Show SMILES CC1(C)Cc2c(c(nn2-c2ccc(C(N)=O)c(N[C@H]3CC[C@H](O)CC3)c2)C(F)(F)F)C(=O)C1 |r,wU:18.18,wD:21.22,(-2.35,-2,;-2.38,-.77,;-3.43,-.12,;-1.03,-1.55,;.3,-.77,;.3,.77,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.68,;3.35,-7.18,;4.53,-7.52,;2.46,-8.03,;4.09,-4.62,;5.57,-5.04,;6.68,-3.97,;6.3,-2.47,;7.41,-1.4,;8.89,-1.82,;9.77,-.96,;9.27,-3.32,;8.16,-4.39,;3.72,-3.12,;2.24,2.7,;3.44,2.95,;1.41,3.61,;2.62,3.87,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,)| Show InChI InChI=1S/C23H27F3N4O3/c1-22(2)10-17-19(18(32)11-22)20(23(24,25)26)29-30(17)13-5-8-15(21(27)33)16(9-13)28-12-3-6-14(31)7-4-12/h5,8-9,12,14,28,31H,3-4,6-7,10-11H2,1-2H3,(H2,27,33)/t12-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human A375 cells assessed as Hsp70 induction after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50008057
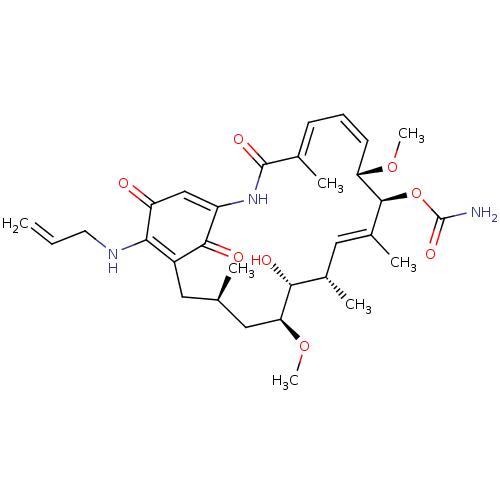
(BMS-722782 | CHEBI:64153 | TANESPIMYCIN)Show SMILES CO[C@H]1C[C@H](C)CC2=C(NCC=C)C(=O)C=C(NC(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)C2=O |r,c:7,23,t:15,21,34| Show InChI InChI=1S/C31H43N3O8/c1-8-12-33-26-21-13-17(2)14-25(41-7)27(36)19(4)15-20(5)29(42-31(32)39)24(40-6)11-9-10-18(3)30(38)34-22(28(21)37)16-23(26)35/h8-11,15-17,19,24-25,27,29,33,36H,1,12-14H2,2-7H3,(H2,32,39)(H,34,38)/b11-9-,18-10+,20-15+/t17-,19+,24+,25+,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human AU565 cells assessed as Her2 degradation after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50008057

(BMS-722782 | CHEBI:64153 | TANESPIMYCIN)Show SMILES CO[C@H]1C[C@H](C)CC2=C(NCC=C)C(=O)C=C(NC(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)C2=O |r,c:7,23,t:15,21,34| Show InChI InChI=1S/C31H43N3O8/c1-8-12-33-26-21-13-17(2)14-25(41-7)27(36)19(4)15-20(5)29(42-31(32)39)24(40-6)11-9-10-18(3)30(38)34-22(28(21)37)16-23(26)35/h8-11,15-17,19,24-25,27,29,33,36H,1,12-14H2,2-7H3,(H2,32,39)(H,34,38)/b11-9-,18-10+,20-15+/t17-,19+,24+,25+,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human A375 cells assessed as Hsp70 induction after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50379853
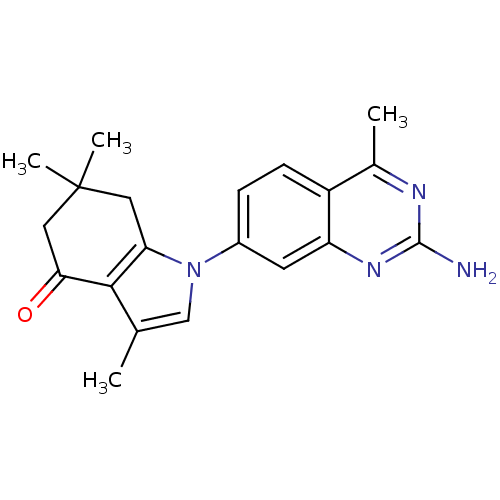
(CHEMBL2011854)Show SMILES Cc1cn(c2CC(C)(C)CC(=O)c12)-c1ccc2c(C)nc(N)nc2c1 Show InChI InChI=1S/C20H22N4O/c1-11-10-24(16-8-20(3,4)9-17(25)18(11)16)13-5-6-14-12(2)22-19(21)23-15(14)7-13/h5-7,10H,8-9H2,1-4H3,(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90-mediated Her2 degradation in human AU565 cells after 24 hrs by ELISA |
Bioorg Med Chem Lett 22: 2550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.137
BindingDB Entry DOI: 10.7270/Q2WQ04TB |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50379853

(CHEMBL2011854)Show SMILES Cc1cn(c2CC(C)(C)CC(=O)c12)-c1ccc2c(C)nc(N)nc2c1 Show InChI InChI=1S/C20H22N4O/c1-11-10-24(16-8-20(3,4)9-17(25)18(11)16)13-5-6-14-12(2)22-19(21)23-15(14)7-13/h5-7,10H,8-9H2,1-4H3,(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human A375 cells assessed as induction of HSP70 synthesis after 24 hrs by TRITC assay |
Bioorg Med Chem Lett 22: 2550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.137
BindingDB Entry DOI: 10.7270/Q2WQ04TB |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50480378
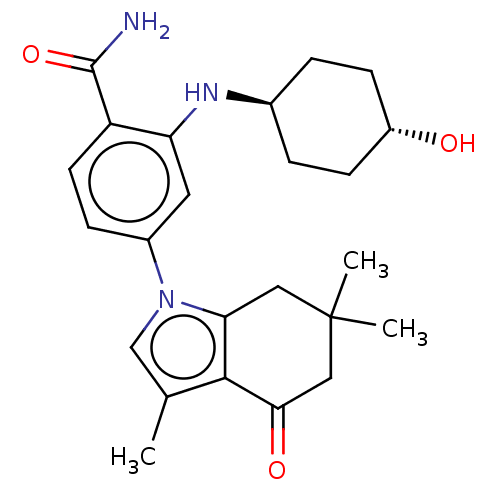
(CHEMBL563608)Show SMILES Cc1cn(c2CC(C)(C)CC(=O)c12)-c1ccc(C(N)=O)c(N[C@H]2CC[C@H](O)CC2)c1 |r,wU:22.23,wD:25.27,(2.14,2.41,;1.76,1.24,;2.66,.02,;1.76,-1.24,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.35,-2,;-3.43,-.12,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.68,;3.35,-7.18,;4.53,-7.52,;2.46,-8.03,;4.09,-4.62,;5.57,-5.04,;6.68,-3.97,;6.3,-2.47,;7.41,-1.4,;8.89,-1.82,;9.77,-.96,;9.27,-3.32,;8.16,-4.39,;3.72,-3.12,)| Show InChI InChI=1S/C24H31N3O3/c1-14-13-27(20-11-24(2,3)12-21(29)22(14)20)16-6-9-18(23(25)30)19(10-16)26-15-4-7-17(28)8-5-15/h6,9-10,13,15,17,26,28H,4-5,7-8,11-12H2,1-3H3,(H2,25,30)/t15-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human A375 cells assessed as pS6 degradation after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50008057

(BMS-722782 | CHEBI:64153 | TANESPIMYCIN)Show SMILES CO[C@H]1C[C@H](C)CC2=C(NCC=C)C(=O)C=C(NC(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)C2=O |r,c:7,23,t:15,21,34| Show InChI InChI=1S/C31H43N3O8/c1-8-12-33-26-21-13-17(2)14-25(41-7)27(36)19(4)15-20(5)29(42-31(32)39)24(40-6)11-9-10-18(3)30(38)34-22(28(21)37)16-23(26)35/h8-11,15-17,19,24-25,27,29,33,36H,1,12-14H2,2-7H3,(H2,32,39)(H,34,38)/b11-9-,18-10+,20-15+/t17-,19+,24+,25+,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human AU565 cells assessed as pERK degradation after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50128790
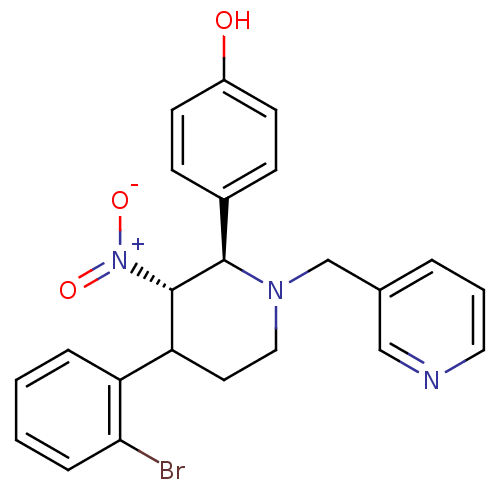
(4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...)Show SMILES Oc1ccc(cc1)[C@@H]1[C@H](C(CCN1Cc1cccnc1)c1ccccc1Br)[N+]([O-])=O Show InChI InChI=1S/C23H22BrN3O3/c24-21-6-2-1-5-19(21)20-11-13-26(15-16-4-3-12-25-14-16)22(23(20)27(29)30)17-7-9-18(28)10-8-17/h1-10,12,14,20,22-23,28H,11,13,15H2/t20?,22-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras |
J Med Chem 46: 2467-73 (2003)
Article DOI: 10.1021/jm020522k
BindingDB Entry DOI: 10.7270/Q2RN3775 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50128790

(4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...)Show SMILES Oc1ccc(cc1)[C@@H]1[C@H](C(CCN1Cc1cccnc1)c1ccccc1Br)[N+]([O-])=O Show InChI InChI=1S/C23H22BrN3O3/c24-21-6-2-1-5-19(21)20-11-13-26(15-16-4-3-12-25-14-16)22(23(20)27(29)30)17-7-9-18(28)10-8-17/h1-10,12,14,20,22-23,28H,11,13,15H2/t20?,22-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras |
J Med Chem 46: 2467-73 (2003)
Article DOI: 10.1021/jm020522k
BindingDB Entry DOI: 10.7270/Q2RN3775 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3152
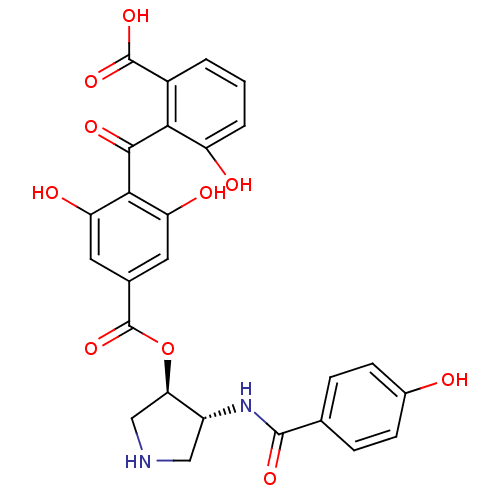
(2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C26H22N2O10/c29-14-6-4-12(5-7-14)24(34)28-16-10-27-11-20(16)38-26(37)13-8-18(31)22(19(32)9-13)23(33)21-15(25(35)36)2-1-3-17(21)30/h1-9,16,20,27,29-32H,10-11H2,(H,28,34)(H,35,36)/t16-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50379853

(CHEMBL2011854)Show SMILES Cc1cn(c2CC(C)(C)CC(=O)c12)-c1ccc2c(C)nc(N)nc2c1 Show InChI InChI=1S/C20H22N4O/c1-11-10-24(16-8-20(3,4)9-17(25)18(11)16)13-5-6-14-12(2)22-19(21)23-15(14)7-13/h5-7,10H,8-9H2,1-4H3,(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90-mediated pS6 phosphorylation in human A375 cells after 24 hrs by TRITC assay |
Bioorg Med Chem Lett 22: 2550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.137
BindingDB Entry DOI: 10.7270/Q2WQ04TB |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50480379
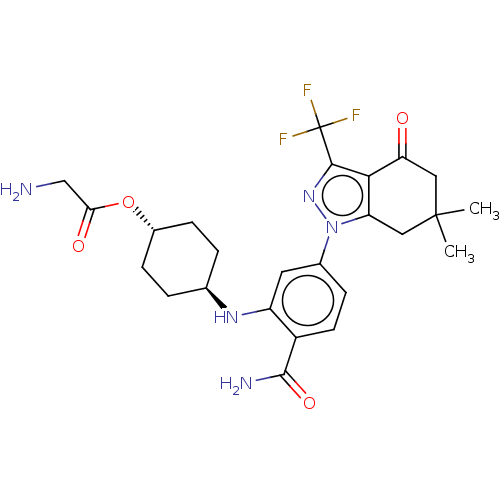
(CHEMBL553939 | SNX-5422)Show SMILES CS(O)(=O)=O.CC1(C)Cc2c(c(nn2-c2ccc(C(N)=O)c(N[C@H]3CC[C@@H](CC3)OC(=O)CN)c2)C(F)(F)F)C(=O)C1 |r,wU:26.29,wD:23.22,(1.33,-.46,;1.33,.77,;1.33,2,;.27,.15,;2.4,.15,;6.56,.62,;6.53,1.85,;5.48,2.5,;7.88,1.07,;9.21,1.85,;9.21,3.39,;10.67,3.86,;11.57,2.64,;10.67,1.39,;11.15,-.08,;10.22,-1.3,;10.82,-2.72,;12.34,-2.91,;12.93,-4.34,;14.16,-4.49,;12.19,-5.31,;13.28,-1.69,;14.81,-1.88,;15.74,-.65,;17.27,-.85,;18.2,.38,;17.6,1.8,;16.07,1.99,;15.14,.76,;18.52,3.03,;17.92,4.45,;16.7,4.6,;18.85,5.68,;18.37,6.82,;12.68,-.27,;11.15,5.32,;12.35,5.58,;10.32,6.24,;11.53,6.49,;7.88,4.18,;7.88,5.41,;6.53,3.39,)| Show InChI InChI=1S/C25H30F3N5O4.CH4O3S/c1-24(2)10-18-21(19(34)11-24)22(25(26,27)28)32-33(18)14-5-8-16(23(30)36)17(9-14)31-13-3-6-15(7-4-13)37-20(35)12-29;1-5(2,3)4/h5,8-9,13,15,31H,3-4,6-7,10-12,29H2,1-2H3,(H2,30,36);1H3,(H,2,3,4)/t13-,15-; | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human AU565 cells assessed as pERK degradation after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50128796
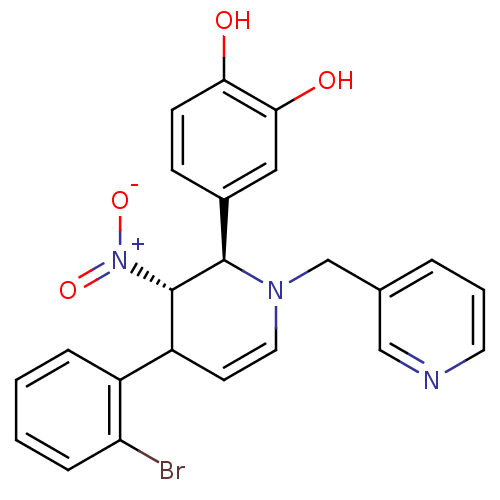
(4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...)Show SMILES Oc1ccc(cc1O)[C@@H]1[C@H](C(C=CN1Cc1cccnc1)c1ccccc1Br)[N+]([O-])=O |c:12| Show InChI InChI=1S/C23H20BrN3O4/c24-19-6-2-1-5-17(19)18-9-11-26(14-15-4-3-10-25-13-15)22(23(18)27(30)31)16-7-8-20(28)21(29)12-16/h1-13,18,22-23,28-29H,14H2/t18?,22-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras |
J Med Chem 46: 2467-73 (2003)
Article DOI: 10.1021/jm020522k
BindingDB Entry DOI: 10.7270/Q2RN3775 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50480379

(CHEMBL553939 | SNX-5422)Show SMILES CS(O)(=O)=O.CC1(C)Cc2c(c(nn2-c2ccc(C(N)=O)c(N[C@H]3CC[C@@H](CC3)OC(=O)CN)c2)C(F)(F)F)C(=O)C1 |r,wU:26.29,wD:23.22,(1.33,-.46,;1.33,.77,;1.33,2,;.27,.15,;2.4,.15,;6.56,.62,;6.53,1.85,;5.48,2.5,;7.88,1.07,;9.21,1.85,;9.21,3.39,;10.67,3.86,;11.57,2.64,;10.67,1.39,;11.15,-.08,;10.22,-1.3,;10.82,-2.72,;12.34,-2.91,;12.93,-4.34,;14.16,-4.49,;12.19,-5.31,;13.28,-1.69,;14.81,-1.88,;15.74,-.65,;17.27,-.85,;18.2,.38,;17.6,1.8,;16.07,1.99,;15.14,.76,;18.52,3.03,;17.92,4.45,;16.7,4.6,;18.85,5.68,;18.37,6.82,;12.68,-.27,;11.15,5.32,;12.35,5.58,;10.32,6.24,;11.53,6.49,;7.88,4.18,;7.88,5.41,;6.53,3.39,)| Show InChI InChI=1S/C25H30F3N5O4.CH4O3S/c1-24(2)10-18-21(19(34)11-24)22(25(26,27)28)32-33(18)14-5-8-16(23(30)36)17(9-14)31-13-3-6-15(7-4-13)37-20(35)12-29;1-5(2,3)4/h5,8-9,13,15,31H,3-4,6-7,10-12,29H2,1-2H3,(H2,30,36);1H3,(H,2,3,4)/t13-,15-; | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human A375 cells assessed as Hsp70 induction after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50480378

(CHEMBL563608)Show SMILES Cc1cn(c2CC(C)(C)CC(=O)c12)-c1ccc(C(N)=O)c(N[C@H]2CC[C@H](O)CC2)c1 |r,wU:22.23,wD:25.27,(2.14,2.41,;1.76,1.24,;2.66,.02,;1.76,-1.24,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.35,-2,;-3.43,-.12,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.68,;3.35,-7.18,;4.53,-7.52,;2.46,-8.03,;4.09,-4.62,;5.57,-5.04,;6.68,-3.97,;6.3,-2.47,;7.41,-1.4,;8.89,-1.82,;9.77,-.96,;9.27,-3.32,;8.16,-4.39,;3.72,-3.12,)| Show InChI InChI=1S/C24H31N3O3/c1-14-13-27(20-11-24(2,3)12-21(29)22(14)20)16-6-9-18(23(25)30)19(10-16)26-15-4-7-17(28)8-5-15/h6,9-10,13,15,17,26,28H,4-5,7-8,11-12H2,1-3H3,(H2,25,30)/t15-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human A375 cells assessed as Hsp70 induction after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50480378

(CHEMBL563608)Show SMILES Cc1cn(c2CC(C)(C)CC(=O)c12)-c1ccc(C(N)=O)c(N[C@H]2CC[C@H](O)CC2)c1 |r,wU:22.23,wD:25.27,(2.14,2.41,;1.76,1.24,;2.66,.02,;1.76,-1.24,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.35,-2,;-3.43,-.12,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.68,;3.35,-7.18,;4.53,-7.52,;2.46,-8.03,;4.09,-4.62,;5.57,-5.04,;6.68,-3.97,;6.3,-2.47,;7.41,-1.4,;8.89,-1.82,;9.77,-.96,;9.27,-3.32,;8.16,-4.39,;3.72,-3.12,)| Show InChI InChI=1S/C24H31N3O3/c1-14-13-27(20-11-24(2,3)12-21(29)22(14)20)16-6-9-18(23(25)30)19(10-16)26-15-4-7-17(28)8-5-15/h6,9-10,13,15,17,26,28H,4-5,7-8,11-12H2,1-3H3,(H2,25,30)/t15-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human AU565 cells assessed as pERK degradation after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3152

(2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C26H22N2O10/c29-14-6-4-12(5-7-14)24(34)28-16-10-27-11-20(16)38-26(37)13-8-18(31)22(19(32)9-13)23(33)21-15(25(35)36)2-1-3-17(21)30/h1-9,16,20,27,29-32H,10-11H2,(H,28,34)(H,35,36)/t16-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50128795
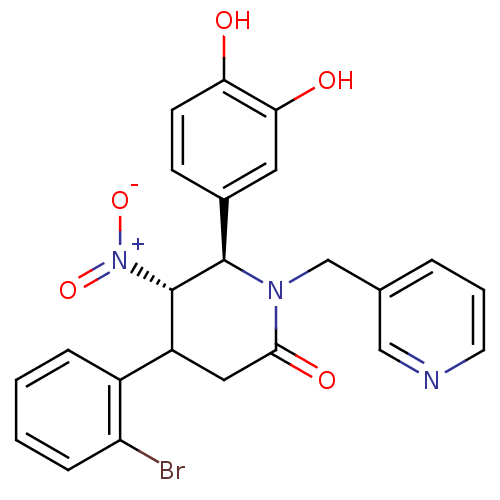
(4-(2-Bromo-phenyl)-6-(3,4-dihydroxy-phenyl)-5-nitr...)Show SMILES Oc1ccc(cc1O)[C@@H]1[C@H](C(CC(=O)N1Cc1cccnc1)c1ccccc1Br)[N+]([O-])=O Show InChI InChI=1S/C23H20BrN3O5/c24-18-6-2-1-5-16(18)17-11-21(30)26(13-14-4-3-9-25-12-14)22(23(17)27(31)32)15-7-8-19(28)20(29)10-15/h1-10,12,17,22-23,28-29H,11,13H2/t17?,22-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras |
J Med Chem 46: 2467-73 (2003)
Article DOI: 10.1021/jm020522k
BindingDB Entry DOI: 10.7270/Q2RN3775 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50008057

(BMS-722782 | CHEBI:64153 | TANESPIMYCIN)Show SMILES CO[C@H]1C[C@H](C)CC2=C(NCC=C)C(=O)C=C(NC(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)C2=O |r,c:7,23,t:15,21,34| Show InChI InChI=1S/C31H43N3O8/c1-8-12-33-26-21-13-17(2)14-25(41-7)27(36)19(4)15-20(5)29(42-31(32)39)24(40-6)11-9-10-18(3)30(38)34-22(28(21)37)16-23(26)35/h8-11,15-17,19,24-25,27,29,33,36H,1,12-14H2,2-7H3,(H2,32,39)(H,34,38)/b11-9-,18-10+,20-15+/t17-,19+,24+,25+,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human A375 cells assessed as pS6 degradation after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3152

(2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C26H22N2O10/c29-14-6-4-12(5-7-14)24(34)28-16-10-27-11-20(16)38-26(37)13-8-18(31)22(19(32)9-13)23(33)21-15(25(35)36)2-1-3-17(21)30/h1-9,16,20,27,29-32H,10-11H2,(H,28,34)(H,35,36)/t16-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50379853

(CHEMBL2011854)Show SMILES Cc1cn(c2CC(C)(C)CC(=O)c12)-c1ccc2c(C)nc(N)nc2c1 Show InChI InChI=1S/C20H22N4O/c1-11-10-24(16-8-20(3,4)9-17(25)18(11)16)13-5-6-14-12(2)22-19(21)23-15(14)7-13/h5-7,10H,8-9H2,1-4H3,(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90-mediated Erk phosphorylation in human AU565 cells after 24 hrs by TRITC assay |
Bioorg Med Chem Lett 22: 2550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.137
BindingDB Entry DOI: 10.7270/Q2WQ04TB |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50450704

(CHEMBL560895 | SNX-2112)Show SMILES CC1(C)Cc2c(c(nn2-c2ccc(C(N)=O)c(N[C@H]3CC[C@H](O)CC3)c2)C(F)(F)F)C(=O)C1 |r,wU:18.18,wD:21.22,(-2.35,-2,;-2.38,-.77,;-3.43,-.12,;-1.03,-1.55,;.3,-.77,;.3,.77,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.68,;3.35,-7.18,;4.53,-7.52,;2.46,-8.03,;4.09,-4.62,;5.57,-5.04,;6.68,-3.97,;6.3,-2.47,;7.41,-1.4,;8.89,-1.82,;9.77,-.96,;9.27,-3.32,;8.16,-4.39,;3.72,-3.12,;2.24,2.7,;3.44,2.95,;1.41,3.61,;2.62,3.87,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,)| Show InChI InChI=1S/C23H27F3N4O3/c1-22(2)10-17-19(18(32)11-22)20(23(24,25)26)29-30(17)13-5-8-15(21(27)33)16(9-13)28-12-3-6-14(31)7-4-12/h5,8-9,12,14,28,31H,3-4,6-7,10-11H2,1-2H3,(H2,27,33)/t12-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human AU565 cells assessed as pERK degradation after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50480374
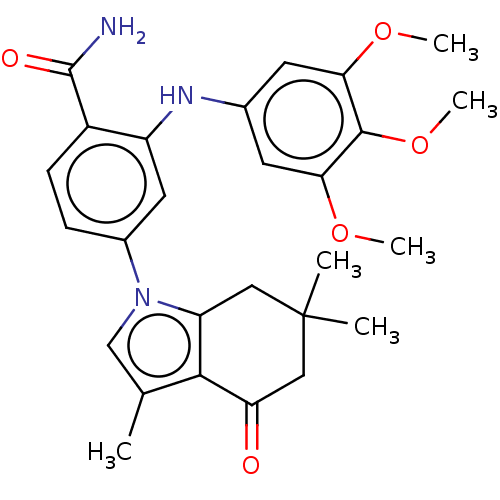
(CHEMBL560955)Show SMILES COc1cc(Nc2cc(ccc2C(N)=O)-n2cc(C)c3c2CC(C)(C)CC3=O)cc(OC)c1OC Show InChI InChI=1S/C27H31N3O5/c1-15-14-30(20-12-27(2,3)13-21(31)24(15)20)17-7-8-18(26(28)32)19(11-17)29-16-9-22(33-4)25(35-6)23(10-16)34-5/h7-11,14,29H,12-13H2,1-6H3,(H2,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human AU565 cells assessed as Her2 degradation after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3230

((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CNC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)ccc3CCCCc23)c(O)c1 |r| Show InChI InChI=1S/C29H28N2O8/c32-18-8-5-16(6-9-18)28(37)31-20-13-30-14-24(20)39-29(38)17-11-22(34)26(23(35)12-17)27(36)25-19-4-2-1-3-15(19)7-10-21(25)33/h5-12,20,24,30,32-35H,1-4,13-14H2,(H,31,37)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3153

(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23NO10/c29-15-9-7-13(8-10-15)25(34)28-17-4-2-6-21(17)38-27(37)14-11-19(31)23(20(32)12-14)24(33)22-16(26(35)36)3-1-5-18(22)30/h1,3,5,7-12,17,21,29-32H,2,4,6H2,(H,28,34)(H,35,36)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3235

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2NS(=O)(=O)C(F)(F)F)c(O)c1 |r| Show InChI InChI=1S/C27H23F3N2O10S/c28-27(29,30)43(40,41)32-17-4-1-5-18(34)22(17)24(37)23-19(35)11-14(12-20(23)36)26(39)42-21-6-2-3-16(21)31-25(38)13-7-9-15(33)10-8-13/h1,4-5,7-12,16,21,32-36H,2-3,6H2,(H,31,38)/t16-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3152

(2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C26H22N2O10/c29-14-6-4-12(5-7-14)24(34)28-16-10-27-11-20(16)38-26(37)13-8-18(31)22(19(32)9-13)23(33)21-15(25(35)36)2-1-3-17(21)30/h1-9,16,20,27,29-32H,10-11H2,(H,28,34)(H,35,36)/t16-,20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50480379

(CHEMBL553939 | SNX-5422)Show SMILES CS(O)(=O)=O.CC1(C)Cc2c(c(nn2-c2ccc(C(N)=O)c(N[C@H]3CC[C@@H](CC3)OC(=O)CN)c2)C(F)(F)F)C(=O)C1 |r,wU:26.29,wD:23.22,(1.33,-.46,;1.33,.77,;1.33,2,;.27,.15,;2.4,.15,;6.56,.62,;6.53,1.85,;5.48,2.5,;7.88,1.07,;9.21,1.85,;9.21,3.39,;10.67,3.86,;11.57,2.64,;10.67,1.39,;11.15,-.08,;10.22,-1.3,;10.82,-2.72,;12.34,-2.91,;12.93,-4.34,;14.16,-4.49,;12.19,-5.31,;13.28,-1.69,;14.81,-1.88,;15.74,-.65,;17.27,-.85,;18.2,.38,;17.6,1.8,;16.07,1.99,;15.14,.76,;18.52,3.03,;17.92,4.45,;16.7,4.6,;18.85,5.68,;18.37,6.82,;12.68,-.27,;11.15,5.32,;12.35,5.58,;10.32,6.24,;11.53,6.49,;7.88,4.18,;7.88,5.41,;6.53,3.39,)| Show InChI InChI=1S/C25H30F3N5O4.CH4O3S/c1-24(2)10-18-21(19(34)11-24)22(25(26,27)28)32-33(18)14-5-8-16(23(30)36)17(9-14)31-13-3-6-15(7-4-13)37-20(35)12-29;1-5(2,3)4/h5,8-9,13,15,31H,3-4,6-7,10-12,29H2,1-2H3,(H2,30,36);1H3,(H,2,3,4)/t13-,15-; | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc
Curated by ChEMBL
| Assay Description
Inhibition of Hsp90 in human A375 cells assessed as pS6 degradation after 24 hrs by high content screening |
J Med Chem 52: 4288-305 (2009)
Article DOI: 10.1021/jm900230j
BindingDB Entry DOI: 10.7270/Q2MK6GQJ |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50128800
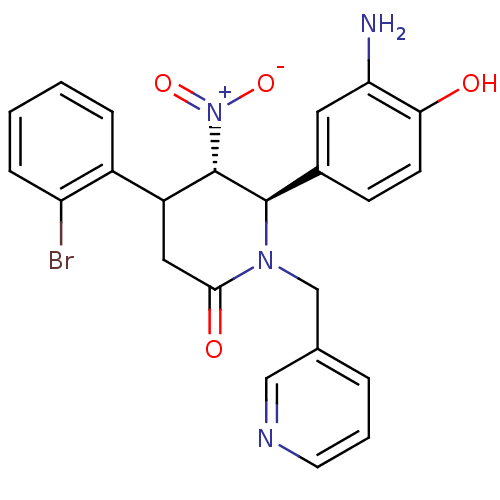
(6-(3-Amino-4-hydroxy-phenyl)-4-(2-bromo-phenyl)-5-...)Show SMILES Nc1cc(ccc1O)[C@@H]1[C@H](C(CC(=O)N1Cc1cccnc1)c1ccccc1Br)[N+]([O-])=O Show InChI InChI=1S/C23H21BrN4O4/c24-18-6-2-1-5-16(18)17-11-21(30)27(13-14-4-3-9-26-12-14)22(23(17)28(31)32)15-7-8-20(29)19(25)10-15/h1-10,12,17,22-23,29H,11,13,25H2/t17?,22-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras |
J Med Chem 46: 2467-73 (2003)
Article DOI: 10.1021/jm020522k
BindingDB Entry DOI: 10.7270/Q2RN3775 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3235

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2NS(=O)(=O)C(F)(F)F)c(O)c1 |r| Show InChI InChI=1S/C27H23F3N2O10S/c28-27(29,30)43(40,41)32-17-4-1-5-18(34)22(17)24(37)23-19(35)11-14(12-20(23)36)26(39)42-21-6-2-3-16(21)31-25(38)13-7-9-15(33)10-8-13/h1,4-5,7-12,16,21,32-36H,2-3,6H2,(H,31,38)/t16-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3236
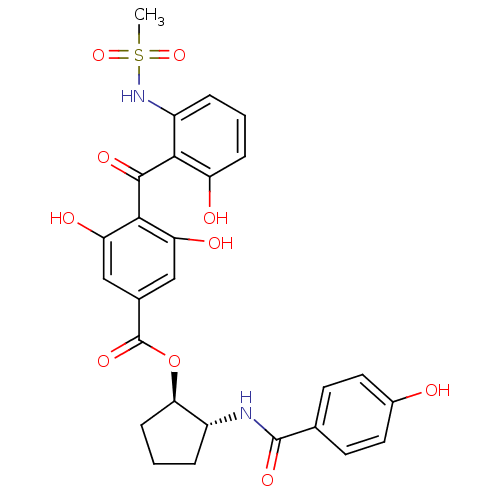
((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES CS(=O)(=O)Nc1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H26N2O10S/c1-40(37,38)29-18-5-2-6-19(31)23(18)25(34)24-20(32)12-15(13-21(24)33)27(36)39-22-7-3-4-17(22)28-26(35)14-8-10-16(30)11-9-14/h2,5-6,8-13,17,22,29-33H,3-4,7H2,1H3,(H,28,35)/t17-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50128789
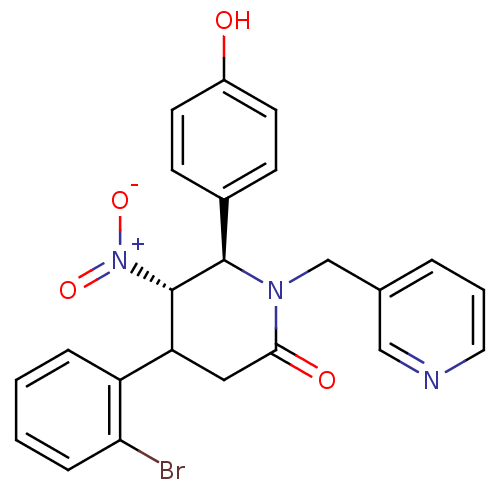
(4-(2-Bromo-phenyl)-6-(4-hydroxy-phenyl)-5-nitro-1-...)Show SMILES Oc1ccc(cc1)[C@@H]1[C@H](C(CC(=O)N1Cc1cccnc1)c1ccccc1Br)[N+]([O-])=O Show InChI InChI=1S/C23H20BrN3O4/c24-20-6-2-1-5-18(20)19-12-21(29)26(14-15-4-3-11-25-13-15)22(23(19)27(30)31)16-7-9-17(28)10-8-16/h1-11,13,19,22-23,28H,12,14H2/t19?,22-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras |
J Med Chem 46: 2467-73 (2003)
Article DOI: 10.1021/jm020522k
BindingDB Entry DOI: 10.7270/Q2RN3775 |
More data for this
Ligand-Target Pair | |
Death-associated protein kinase 1
(Homo sapiens (Human)) | BDBM104065
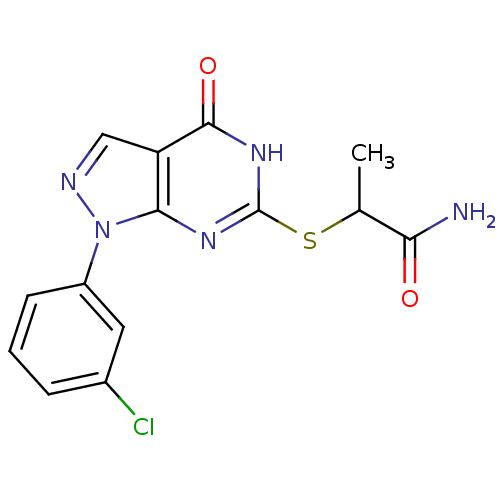
(HS38)Show SMILES CC(Sc1nc2n(ncc2c(=O)[nH]1)-c1cccc(Cl)c1)C(N)=O Show InChI InChI=1S/C14H12ClN5O2S/c1-7(11(16)21)23-14-18-12-10(13(22)19-14)6-17-20(12)9-4-2-3-8(15)5-9/h2-7H,1H3,(H2,16,21)(H,18,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
| Assay Description
HS38 was evaluated using a P-33 ATP filter-binding assay by the International Centre for Kinase Profiling (University of Dundee) against 124 purified... |
ACS Chem Biol 8: 2715-23 (2013)
Article DOI: 10.1021/cb400407c
BindingDB Entry DOI: 10.7270/Q24B2ZXR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM104065

(HS38)Show SMILES CC(Sc1nc2n(ncc2c(=O)[nH]1)-c1cccc(Cl)c1)C(N)=O Show InChI InChI=1S/C14H12ClN5O2S/c1-7(11(16)21)23-14-18-12-10(13(22)19-14)6-17-20(12)9-4-2-3-8(15)5-9/h2-7H,1H3,(H2,16,21)(H,18,19,22) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center
| Assay Description
HS38 was evaluated using a P-33 ATP filter-binding assay by the International Centre for Kinase Profiling (University of Dundee) against 124 purified... |
ACS Chem Biol 8: 2715-23 (2013)
Article DOI: 10.1021/cb400407c
BindingDB Entry DOI: 10.7270/Q24B2ZXR |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3239
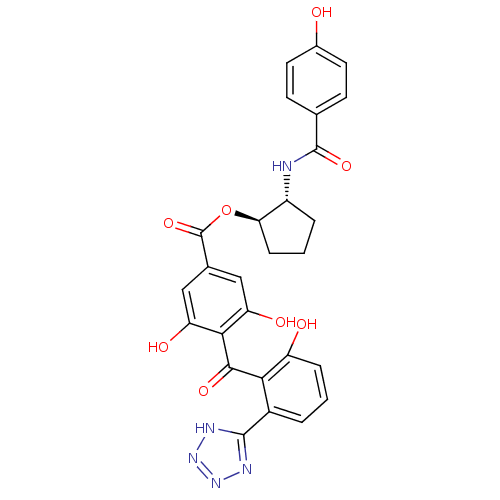
((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2-c2nnn[nH]2)c(O)c1 |r| Show InChI InChI=1S/C27H23N5O8/c33-15-9-7-13(8-10-15)26(38)28-17-4-2-6-21(17)40-27(39)14-11-19(35)23(20(36)12-14)24(37)22-16(3-1-5-18(22)34)25-29-31-32-30-25/h1,3,5,7-12,17,21,33-36H,2,4,6H2,(H,28,38)(H,29,30,31,32)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3231

((3R,4R)-3-[(4-hydroxybenzene)amido]azepan-4-yl 3,5...)Show SMILES COc1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H28N2O9/c1-38-23-5-2-4-19(32)25(23)26(35)24-20(33)12-16(13-21(24)34)28(37)39-22-6-3-11-29-14-18(22)30-27(36)15-7-9-17(31)10-8-15/h2,4-5,7-10,12-13,18,22,29,31-34H,3,6,11,14H2,1H3,(H,30,36)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3230

((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CNC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)ccc3CCCCc23)c(O)c1 |r| Show InChI InChI=1S/C29H28N2O8/c32-18-8-5-16(6-9-18)28(37)31-20-13-30-14-24(20)39-29(38)17-11-22(34)26(23(35)12-17)27(36)25-19-4-2-1-3-15(19)7-10-21(25)33/h5-12,20,24,30,32-35H,1-4,13-14H2,(H,31,37)/t20-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3239

((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2-c2nnn[nH]2)c(O)c1 |r| Show InChI InChI=1S/C27H23N5O8/c33-15-9-7-13(8-10-15)26(38)28-17-4-2-6-21(17)40-27(39)14-11-19(35)23(20(36)12-14)24(37)22-16(3-1-5-18(22)34)25-29-31-32-30-25/h1,3,5,7-12,17,21,33-36H,2,4,6H2,(H,28,38)(H,29,30,31,32)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3220
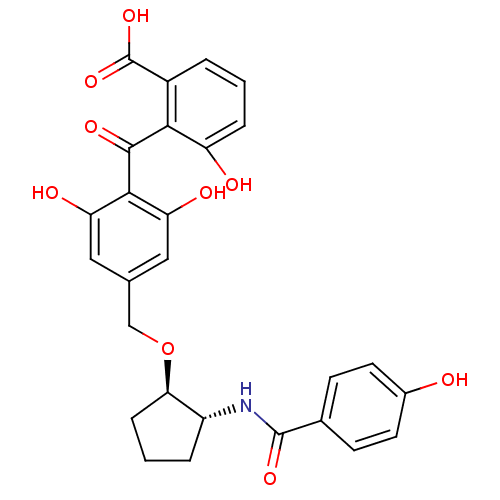
(2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(CO[C@@H]2CCC[C@H]2NC(=O)c2ccc(O)cc2)cc1O |r| Show InChI InChI=1S/C27H25NO9/c29-16-9-7-15(8-10-16)26(34)28-18-4-2-6-22(18)37-13-14-11-20(31)24(21(32)12-14)25(33)23-17(27(35)36)3-1-5-19(23)30/h1,3,5,7-12,18,22,29-32H,2,4,6,13H2,(H,28,34)(H,35,36)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3228

((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CNC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)ccc3ccccc23)c(O)c1 |r| Show InChI InChI=1S/C29H24N2O8/c32-18-8-5-16(6-9-18)28(37)31-20-13-30-14-24(20)39-29(38)17-11-22(34)26(23(35)12-17)27(36)25-19-4-2-1-3-15(19)7-10-21(25)33/h1-12,20,24,30,32-35H,13-14H2,(H,31,37)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM3226
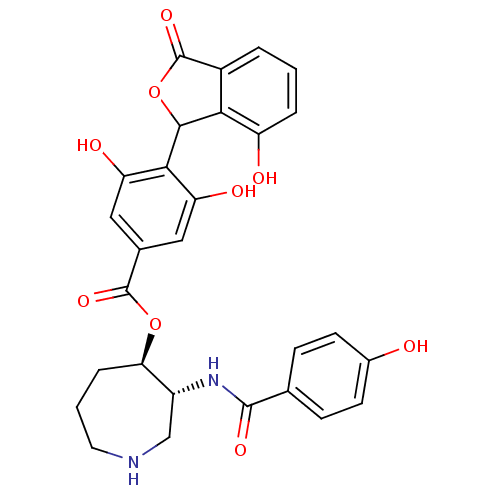
((3R,4R)-3-[(4-hydroxybenzene)amido]azepan-4-yl 3,5...)Show SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CNCCC[C@H]1OC(=O)c1cc(O)c(C2OC(=O)c3cccc(O)c23)c(O)c1 |r| Show InChI InChI=1S/C28H26N2O9/c31-16-8-6-14(7-9-16)26(35)30-18-13-29-10-2-5-22(18)38-27(36)15-11-20(33)24(21(34)12-15)25-23-17(28(37)39-25)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,25,29,31-34H,2,5,10,13H2,(H,30,35)/t18-,22-,25?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories
| Assay Description
The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. |
J Med Chem 45: 2624-43 (2002)
Article DOI: 10.1021/jm020018f
BindingDB Entry DOI: 10.7270/Q2BG2M50 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data