

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017519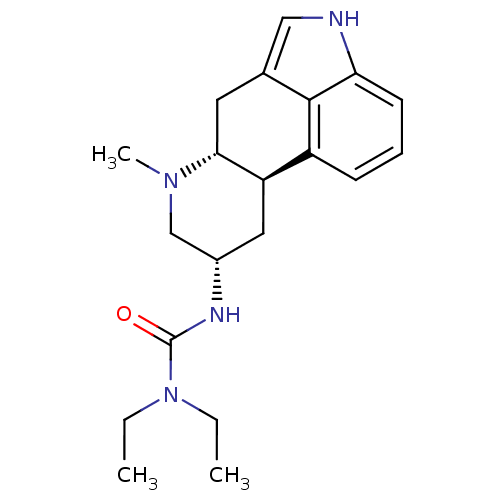 (1,1-Diethyl-3-(7-methyl-4,6,6a,7,8,9,10,10a-octahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against dopamine receptor D2 from homogenized rat brain, using [3H]spiperone as the radioligand | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against 5-hydroxytryptamine 1A receptor receptor from homogenized rat brain, using [3H]8-OH-DPAT as the radioligand | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017519 (1,1-Diethyl-3-(7-methyl-4,6,6a,7,8,9,10,10a-octahy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against 5-hydroxytryptamine 1A receptor from homogenized rat brain, using [3H]-8-OH-DPAT as the radioligand | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50044236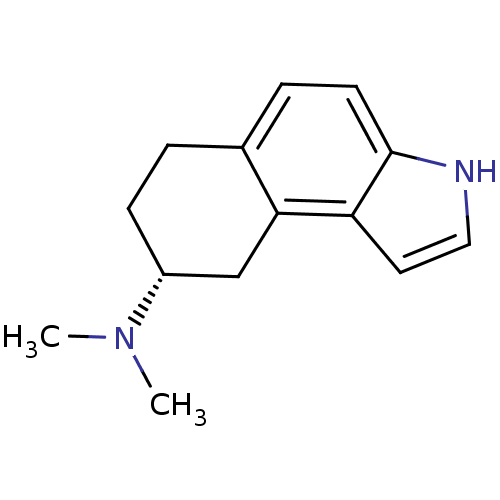 (CHEMBL303339 | Dimethyl-(R)-6,7,8,9-tetrahydro-3H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against 5-hydroxytryptamine 1A receptor from homogenized rat brain, using [3H]8-OH-DPAT as the radioligand. | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM16314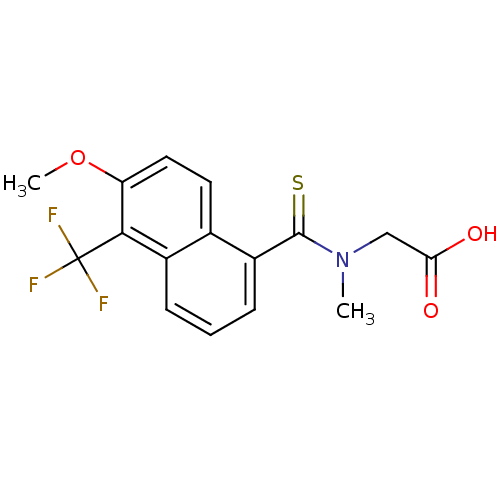 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of bovine lens aldose reductase | J Med Chem 32: 757-65 (1989) BindingDB Entry DOI: 10.7270/Q22B8X0W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of bovine lens aldose reductase with DL-glyceraldehyde as substrate | J Med Chem 27: 255-6 (1984) BindingDB Entry DOI: 10.7270/Q2QR4W4S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50017519 (1,1-Diethyl-3-(7-methyl-4,6,6a,7,8,9,10,10a-octahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against Dopamine receptor D1 from homogenized rat brain, using [3H]SCH-23390 as the radioligand | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017520 ((+/-)-Dimethyl-(6,7,8,9-tetrahydro-3H-benzo[e]indo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against 5-hydroxytryptamine 1A receptor from homogenized rat brain, using [3H]8-OH-DPAT as the radioligand. | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50044235 (CHEMBL292274 | Dimethyl-(S)-6,7,8,9-tetrahydro-3H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against 5-hydroxytryptamine 1A receptor from homogenized rat brain, using [3H]8-OH-DPAT as the radioligand. | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50016901 ((2-Methyl-3-oxo-3H-phenalen-1-yl)-acetic acid | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of bovine lens aldose reductase | J Med Chem 32: 757-65 (1989) BindingDB Entry DOI: 10.7270/Q22B8X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of aldose reductase (or polyol accumulation) in isolated rat sciatic nerve by compound at 10e-5 M concentration | J Med Chem 32: 757-65 (1989) BindingDB Entry DOI: 10.7270/Q22B8X0W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50016902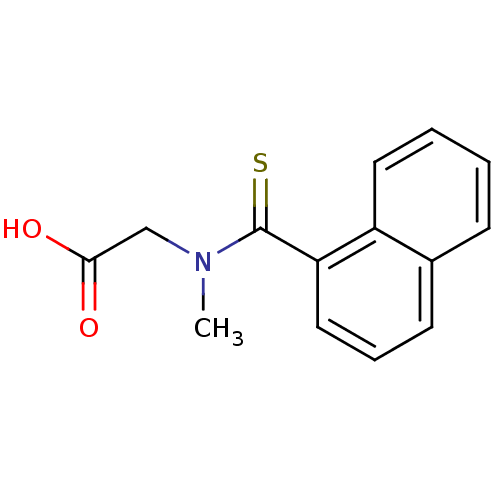 (CHEMBL168106 | [Methyl-(naphthalene-1-carbothioyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of bovine lens aldose reductase | J Med Chem 32: 757-65 (1989) BindingDB Entry DOI: 10.7270/Q22B8X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50044236 (CHEMBL303339 | Dimethyl-(R)-6,7,8,9-tetrahydro-3H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against dopamine receptor D2 from homogenized rat brain, using [3H]spiperone as the radioligand. | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of bovine lens aldose reductase with DL-glyceraldehyde as substrate | J Med Chem 27: 255-6 (1984) BindingDB Entry DOI: 10.7270/Q2QR4W4S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017520 ((+/-)-Dimethyl-(6,7,8,9-tetrahydro-3H-benzo[e]indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against dopamine receptor D2 from homogenized rat brain, using [3H]spiperone as the radioligand. | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50016901 ((2-Methyl-3-oxo-3H-phenalen-1-yl)-acetic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of aldose reductase (or polyol accumulation) in isolated rat sciatic nerve by compound at (10E-5) M concentration | J Med Chem 32: 757-65 (1989) BindingDB Entry DOI: 10.7270/Q22B8X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM16415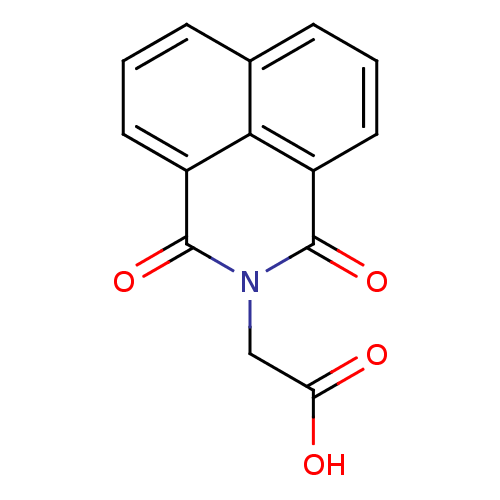 ((1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetic...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of bovine lens aldose reductase with DL-glyceraldehyde as substrate | J Med Chem 27: 255-6 (1984) BindingDB Entry DOI: 10.7270/Q2QR4W4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against Dopamine receptor D2 from homogenized rat brain, using [3H]spiperone as the radioligand | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50044235 (CHEMBL292274 | Dimethyl-(S)-6,7,8,9-tetrahydro-3H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against dopamine receptor D2 from homogenized rat brain, using [3H]spiperone as the radioligand. | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50017520 ((+/-)-Dimethyl-(6,7,8,9-tetrahydro-3H-benzo[e]indo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against dopamine receptor D1 from homogenized rat brain, using [3H]SCH-23390 as the radioligand. | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50044236 (CHEMBL303339 | Dimethyl-(R)-6,7,8,9-tetrahydro-3H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against dopamine receptor D1 from homogenized rat brain, using [3H]SCH-23390 as the radioligand. | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50044235 (CHEMBL292274 | Dimethyl-(S)-6,7,8,9-tetrahydro-3H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against dopamine receptor D1 from homogenized rat brain, using [3H]SCH-23390 as the radioligand. | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£teborg Curated by ChEMBL | Assay Description In vitro binding activity against dopamine D1 receptor from homogenized rat brain, using [3H]SCH-23390 as the radioligand | J Med Chem 32: 2273-6 (1989) BindingDB Entry DOI: 10.7270/Q2KD1ZG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||