 Found 798 hits with Last Name = 'hunsaker' and Initial = 't'
Found 798 hits with Last Name = 'hunsaker' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557772
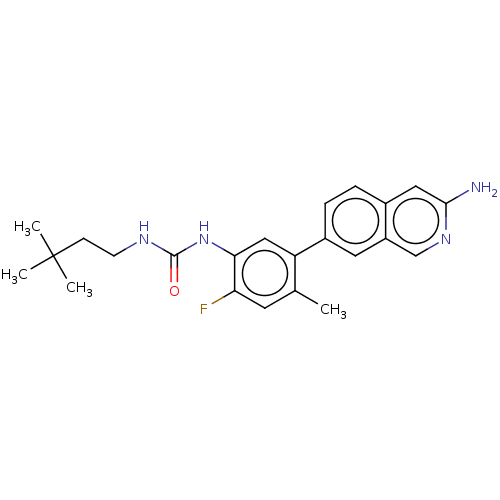
(CHEMBL4775998)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2cc(N)ncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580084
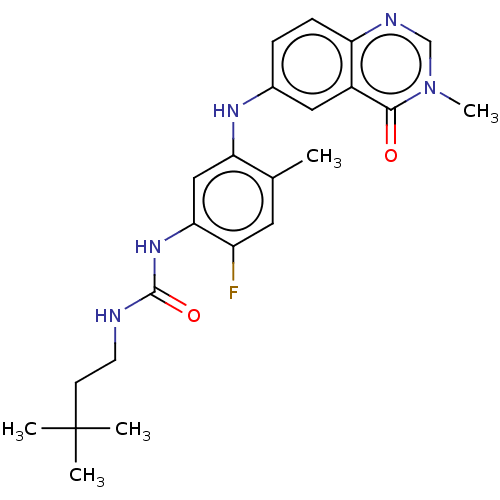
(CHEMBL5075174)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580082
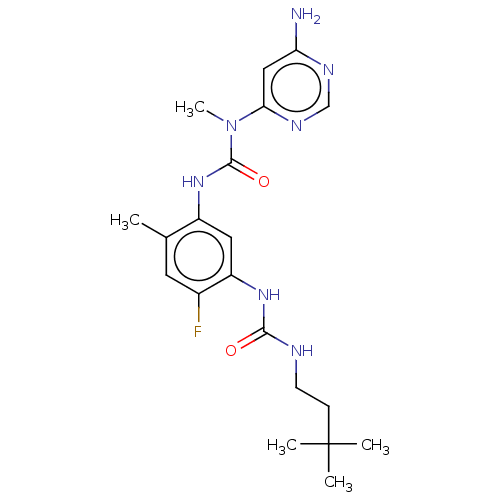
(CHEMBL5079215)Show SMILES CN(C(=O)Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C)c1cc(N)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557773
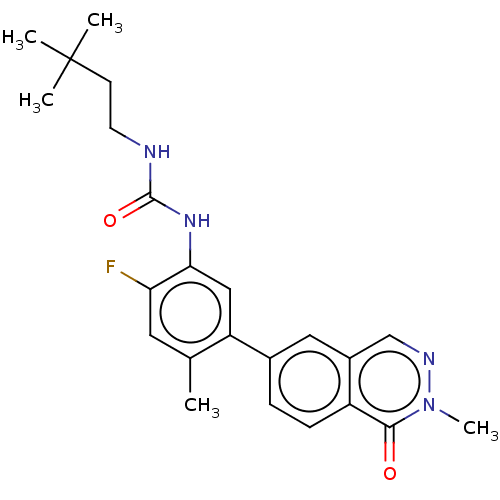
(CHEMBL4778772)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557775
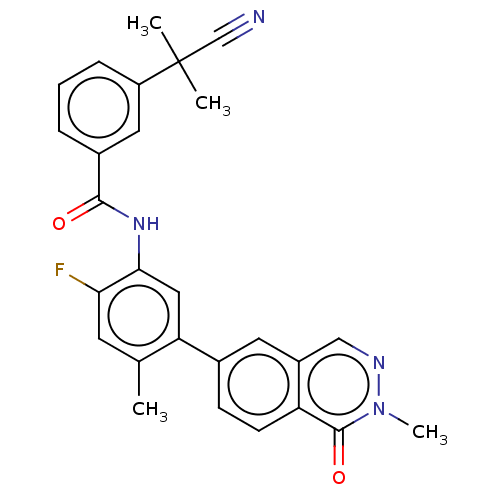
(CHEMBL4758903)Show SMILES Cc1cc(F)c(NC(=O)c2cccc(c2)C(C)(C)C#N)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580080
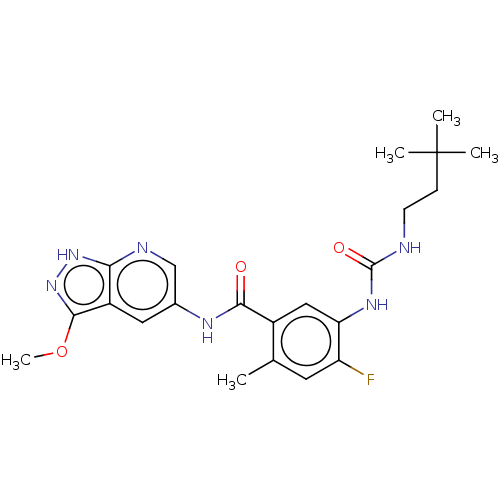
(CHEMBL5090624)Show SMILES COc1n[nH]c2ncc(NC(=O)c3cc(NC(=O)NCCC(C)(C)C)c(F)cc3C)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580083
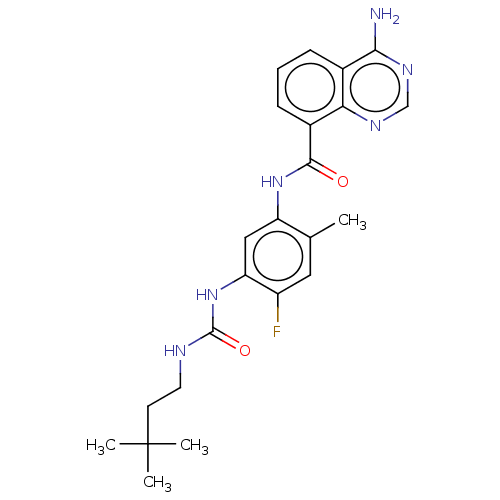
(CHEMBL5094268)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1NC(=O)c1cccc2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557774
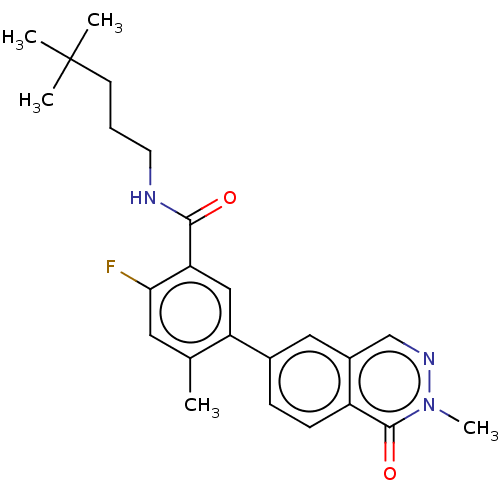
(CHEMBL4776565)Show SMILES Cc1cc(F)c(cc1-c1ccc2c(cnn(C)c2=O)c1)C(=O)NCCCC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580081
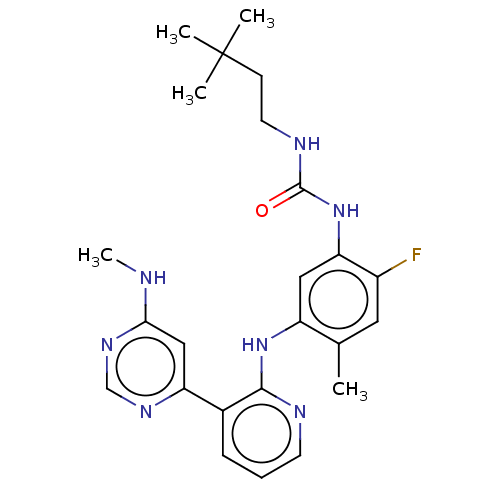
(CHEMBL5094514)Show SMILES CNc1cc(ncn1)-c1cccnc1Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50096279
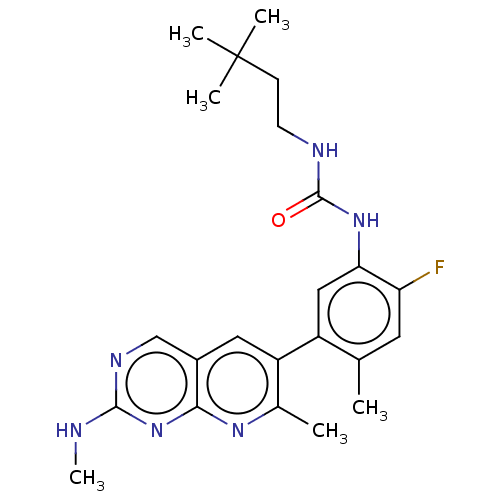
(CHEMBL3577124)Show SMILES CNc1ncc2cc(c(C)nc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C18H13N3O5/c22-14(23)5-4-10-8-21-13-7-12-9(6-15(24)25)2-1-3-11(12)16(13)20-18(26)17(21)19-10/h1-5,8H,6-7H2,(H,20,26)(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50096279

(CHEMBL3577124)Show SMILES CNc1ncc2cc(c(C)nc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C18H13N3O5/c22-14(23)5-4-10-8-21-13-7-12-9(6-15(24)25)2-1-3-11(12)16(13)20-18(26)17(21)19-10/h1-5,8H,6-7H2,(H,20,26)(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557776
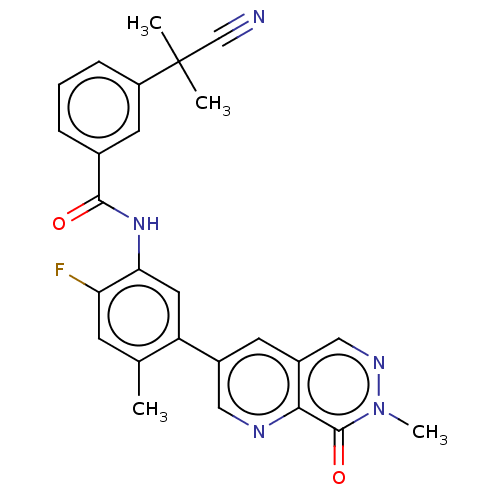
(CHEMBL4778419)Show SMILES Cc1cc(F)c(NC(=O)c2cccc(c2)C(C)(C)C#N)cc1-c1cnc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557775

(CHEMBL4758903)Show SMILES Cc1cc(F)c(NC(=O)c2cccc(c2)C(C)(C)C#N)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557773

(CHEMBL4778772)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557772

(CHEMBL4775998)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2cc(N)ncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580084

(CHEMBL5075174)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580083

(CHEMBL5094268)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1NC(=O)c1cccc2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580082

(CHEMBL5079215)Show SMILES CN(C(=O)Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C)c1cc(N)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580081

(CHEMBL5094514)Show SMILES CNc1cc(ncn1)-c1cccnc1Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557771
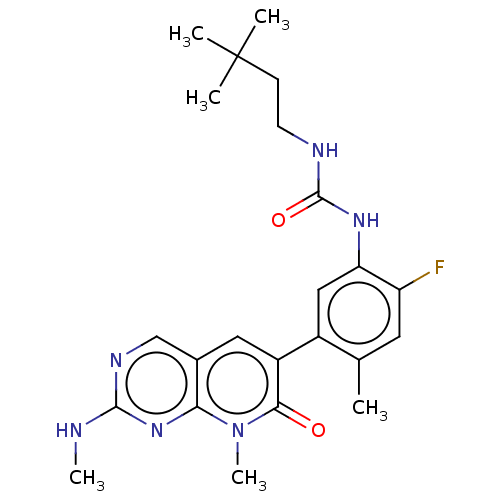
(CHEMBL4740241)Show SMILES CNc1ncc2cc(-c3cc(NC(=O)NCCC(C)(C)C)c(F)cc3C)c(=O)n(C)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557771

(CHEMBL4740241)Show SMILES CNc1ncc2cc(-c3cc(NC(=O)NCCC(C)(C)C)c(F)cc3C)c(=O)n(C)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557774

(CHEMBL4776565)Show SMILES Cc1cc(F)c(cc1-c1ccc2c(cnn(C)c2=O)c1)C(=O)NCCCC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50096279

(CHEMBL3577124)Show SMILES CNc1ncc2cc(c(C)nc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C18H13N3O5/c22-14(23)5-4-10-8-21-13-7-12-9(6-15(24)25)2-1-3-11(12)16(13)20-18(26)17(21)19-10/h1-5,8H,6-7H2,(H,20,26)(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50096279

(CHEMBL3577124)Show SMILES CNc1ncc2cc(c(C)nc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C18H13N3O5/c22-14(23)5-4-10-8-21-13-7-12-9(6-15(24)25)2-1-3-11(12)16(13)20-18(26)17(21)19-10/h1-5,8H,6-7H2,(H,20,26)(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580080

(CHEMBL5090624)Show SMILES COc1n[nH]c2ncc(NC(=O)c3cc(NC(=O)NCCC(C)(C)C)c(F)cc3C)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557776

(CHEMBL4778419)Show SMILES Cc1cc(F)c(NC(=O)c2cccc(c2)C(C)(C)C#N)cc1-c1cnc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532351

(CHEMBL4565958)Show SMILES Cc1n[nH]cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-12-18(10-25-28-12)27-21-24-6-4-17(26-21)13-5-7-29(20(31)9-13)19(11-30)14-2-3-15(22)16(23)8-14/h2-10,19,30H,11H2,1H3,(H,25,28)(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532351

(CHEMBL4565958)Show SMILES Cc1n[nH]cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-12-18(10-25-28-12)27-21-24-6-4-17(26-21)13-5-7-29(20(31)9-13)19(11-30)14-2-3-15(22)16(23)8-14/h2-10,19,30H,11H2,1H3,(H,25,28)(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532350

(CHEMBL4571067)Show SMILES Cc1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)ccn1 |r| Show InChI InChI=1S/C23H19ClFN5O2/c1-14-10-17(4-7-26-14)28-23-27-8-5-20(29-23)15-6-9-30(22(32)12-15)21(13-31)16-2-3-18(24)19(25)11-16/h2-12,21,31H,13H2,1H3,(H,26,27,28,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50269847
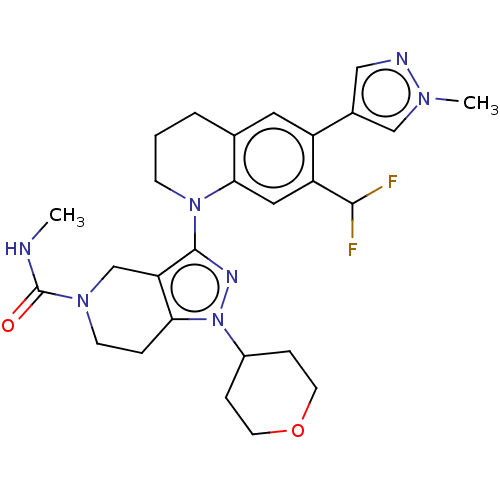
(CHEMBL4097025)Show SMILES CNC(=O)N1CCc2c(C1)c(nn2C1CCOCC1)N1CCCc2cc(-c3cnn(C)c3)c(cc12)C(F)F Show InChI InChI=1S/C27H33F2N7O2/c1-30-27(37)34-9-5-23-22(16-34)26(32-36(23)19-6-10-38-11-7-19)35-8-3-4-17-12-20(18-14-31-33(2)15-18)21(25(28)29)13-24(17)35/h12-15,19,25H,3-11,16H2,1-2H3,(H,30,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532350

(CHEMBL4571067)Show SMILES Cc1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)ccn1 |r| Show InChI InChI=1S/C23H19ClFN5O2/c1-14-10-17(4-7-26-14)28-23-27-8-5-20(29-23)15-6-9-30(22(32)12-15)21(13-31)16-2-3-18(24)19(25)11-16/h2-12,21,31H,13H2,1H3,(H,26,27,28,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50269857
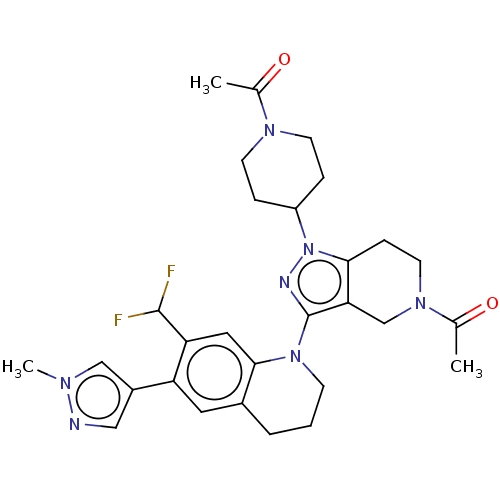
(CHEMBL4088793)Show SMILES CC(=O)N1CCC(CC1)n1nc(N2CCCc3cc(-c4cnn(C)c4)c(cc23)C(F)F)c2CN(CCc12)C(C)=O Show InChI InChI=1S/C29H35F2N7O2/c1-18(39)35-10-6-22(7-11-35)38-26-8-12-36(19(2)40)17-25(26)29(33-38)37-9-4-5-20-13-23(21-15-32-34(3)16-21)24(28(30)31)14-27(20)37/h13-16,22,28H,4-12,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50269848
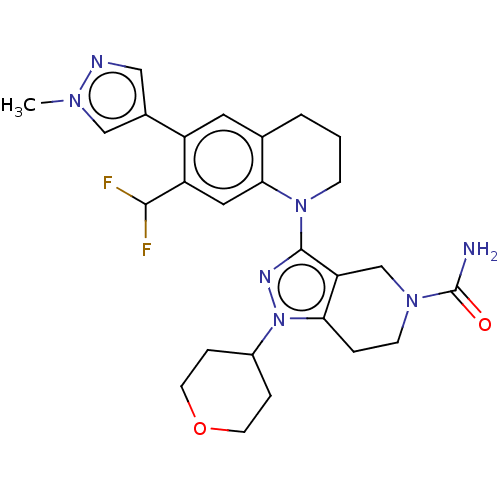
(CHEMBL4069831)Show SMILES Cn1cc(cn1)-c1cc2CCCN(c3nn(C4CCOCC4)c4CCN(Cc34)C(N)=O)c2cc1C(F)F Show InChI InChI=1S/C26H31F2N7O2/c1-32-14-17(13-30-32)19-11-16-3-2-7-34(23(16)12-20(19)24(27)28)25-21-15-33(26(29)36)8-4-22(21)35(31-25)18-5-9-37-10-6-18/h11-14,18,24H,2-10,15H2,1H3,(H2,29,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31093

(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech
| Assay Description
Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... |
J Med Chem 52: 3300-7 (2009)
Article DOI: 10.1021/jm9000314
BindingDB Entry DOI: 10.7270/Q2M90706 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50269849

(CHEMBL4076748)Show SMILES CC(=O)N1CCc2c(C1)c(nn2C1CCOCC1)N1CCCc2cc(-c3cnn(C)c3)c(cc12)C(F)F Show InChI InChI=1S/C27H32F2N6O2/c1-17(36)33-9-5-24-23(16-33)27(31-35(24)20-6-10-37-11-7-20)34-8-3-4-18-12-21(19-14-30-32(2)15-19)22(26(28)29)13-25(18)34/h12-15,20,26H,3-11,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM120086
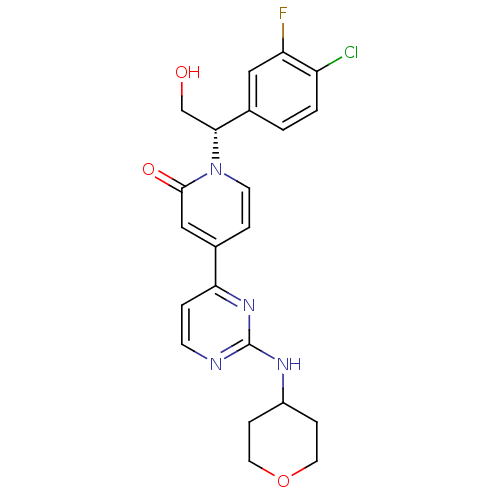
(US8697715, 1 | US9259470, 1)Show SMILES OC[C@H](c1ccc(Cl)c(F)c1)n1ccc(cc1=O)-c1ccnc(NC2CCOCC2)n1 |r| Show InChI InChI=1S/C22H22ClFN4O3/c23-17-2-1-15(11-18(17)24)20(13-29)28-8-4-14(12-21(28)30)19-3-7-25-22(27-19)26-16-5-9-31-10-6-16/h1-4,7-8,11-12,16,20,29H,5-6,9-10,13H2,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM120086

(US8697715, 1 | US9259470, 1)Show SMILES OC[C@H](c1ccc(Cl)c(F)c1)n1ccc(cc1=O)-c1ccnc(NC2CCOCC2)n1 |r| Show InChI InChI=1S/C22H22ClFN4O3/c23-17-2-1-15(11-18(17)24)20(13-29)28-8-4-14(12-21(28)30)19-3-7-25-22(27-19)26-16-5-9-31-10-6-16/h1-4,7-8,11-12,16,20,29H,5-6,9-10,13H2,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50269847

(CHEMBL4097025)Show SMILES CNC(=O)N1CCc2c(C1)c(nn2C1CCOCC1)N1CCCc2cc(-c3cnn(C)c3)c(cc12)C(F)F Show InChI InChI=1S/C27H33F2N7O2/c1-30-27(37)34-9-5-23-22(16-34)26(32-36(23)19-6-10-38-11-7-19)35-8-3-4-17-12-20(18-14-31-33(2)15-18)21(25(28)29)13-24(17)35/h12-15,19,25H,3-11,16H2,1-2H3,(H,30,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged P300 measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50269855

(CHEMBL4061161)Show SMILES CC(=O)N1CCc2c(C1)c(nn2C1CCS(=O)(=O)CC1)N1CCCc2cc(-c3cnn(C)c3)c(cc12)C(F)F Show InChI InChI=1S/C27H32F2N6O3S/c1-17(36)33-9-5-24-23(16-33)27(31-35(24)20-6-10-39(37,38)11-7-20)34-8-3-4-18-12-21(19-14-30-32(2)15-19)22(26(28)29)13-25(18)34/h12-15,20,26H,3-11,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50269856
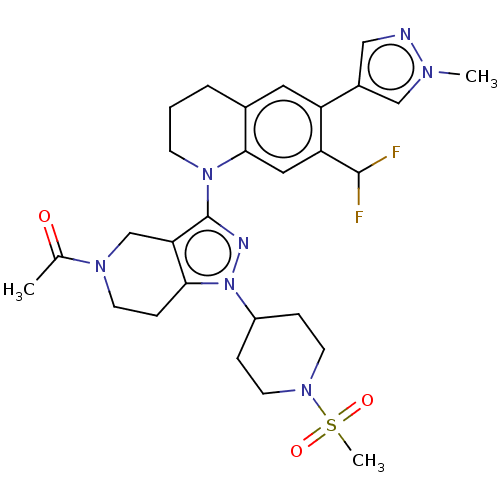
(CHEMBL4060566)Show SMILES CC(=O)N1CCc2c(C1)c(nn2C1CCN(CC1)S(C)(=O)=O)N1CCCc2cc(-c3cnn(C)c3)c(cc12)C(F)F Show InChI InChI=1S/C28H35F2N7O3S/c1-18(38)34-10-8-25-24(17-34)28(32-37(25)21-6-11-35(12-7-21)41(3,39)40)36-9-4-5-19-13-22(20-15-31-33(2)16-20)23(27(29)30)14-26(19)36/h13-16,21,27H,4-12,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50269849

(CHEMBL4076748)Show SMILES CC(=O)N1CCc2c(C1)c(nn2C1CCOCC1)N1CCCc2cc(-c3cnn(C)c3)c(cc12)C(F)F Show InChI InChI=1S/C27H32F2N6O2/c1-17(36)33-9-5-24-23(16-33)27(31-35(24)20-6-10-37-11-7-20)34-8-3-4-18-12-21(19-14-30-32(2)15-19)22(26(28)29)13-25(18)34/h12-15,20,26H,3-11,16H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged P300 measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50269850

(CHEMBL4061600)Show SMILES CC(=O)N1CCc2c(C1)c(nn2[C@H]1CCOC1)N1CCCc2cc(-c3cnn(C)c3)c(cc12)C(F)F |r| Show InChI InChI=1S/C26H30F2N6O2/c1-16(35)32-8-5-23-22(14-32)26(30-34(23)19-6-9-36-15-19)33-7-3-4-17-10-20(18-12-29-31(2)13-18)21(25(27)28)11-24(17)33/h10-13,19,25H,3-9,14-15H2,1-2H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50269907

(CHEMBL4080905)Show SMILES CC(=O)N1CCc2c(C1)c(nn2C1CCNCC1)N1CCCc2cc(-c3cnn(C)c3)c(cc12)C(F)F Show InChI InChI=1S/C27H33F2N7O/c1-17(37)34-11-7-24-23(16-34)27(32-36(24)20-5-8-30-9-6-20)35-10-3-4-18-12-21(19-14-31-33(2)15-19)22(26(28)29)13-25(18)35/h12-15,20,26,30H,3-11,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50269914

(CHEMBL4083416)Show SMILES CC(=O)N1CCc2c(C1)c(nn2[C@H]1CCOC1)N1CCCc2cc(-c3cnn(C)c3)c(cc12)C(F)(F)F |r| Show InChI InChI=1S/C26H29F3N6O2/c1-16(36)33-8-5-23-21(14-33)25(31-35(23)19-6-9-37-15-19)34-7-3-4-17-10-20(18-12-30-32(2)13-18)22(11-24(17)34)26(27,28)29/h10-13,19H,3-9,14-15H2,1-2H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Displacement of biotinylated histone H3K14 peptide ligand from human recombinant His-tagged CBP measured after 10 mins by TR-FRET assay |
J Med Chem 60: 9162-9183 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00796
BindingDB Entry DOI: 10.7270/Q23F4S4V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532362

(CHEMBL4436718)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-2-29-12-16(11-26-29)27-22-25-7-5-19(28-22)14-6-8-30(21(32)10-14)20(13-31)15-3-4-17(23)18(24)9-15/h3-12,20,31H,2,13H2,1H3,(H,25,27,28)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532362

(CHEMBL4436718)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-2-29-12-16(11-26-29)27-22-25-7-5-19(28-22)14-6-8-30(21(32)10-14)20(13-31)15-3-4-17(23)18(24)9-15/h3-12,20,31H,2,13H2,1H3,(H,25,27,28)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data