

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50309502 (CHEMBL591279 | rac-3-(3-(fluoromethyl)phenyl)-9,10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 20: 1106-8 (2010) Article DOI: 10.1016/j.bmcl.2009.12.025 BindingDB Entry DOI: 10.7270/Q2SJ1KQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM511554 (US11059794, Example 6.14 | tert-butyl 4-(2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258501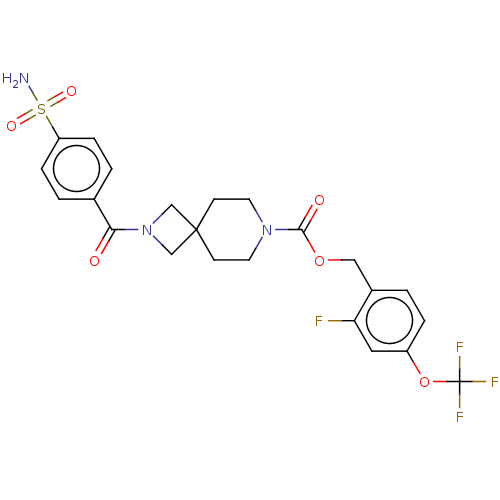 (US10633384, Example 1.23 | US9493486, 1.23) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258574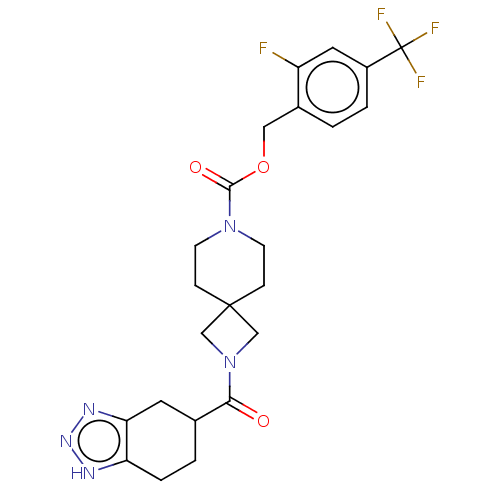 (US10633384, Example 12.36 | US9493486, 12.36) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258575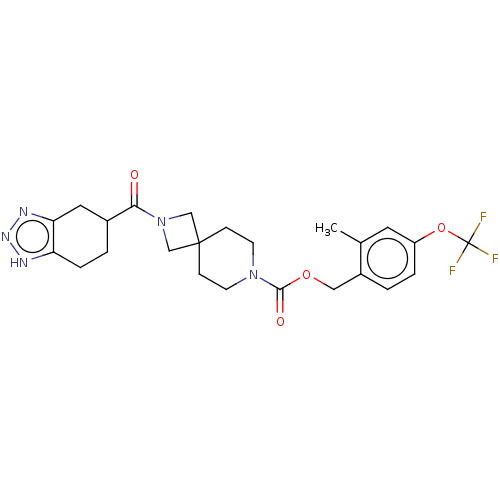 (US10633384, Example 12.37 | US9493486, 12.37) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258580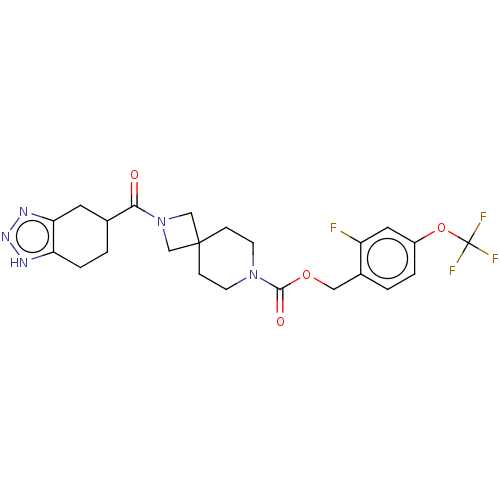 (US10633384, Example 12.42 | US9493486, 12.42) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258581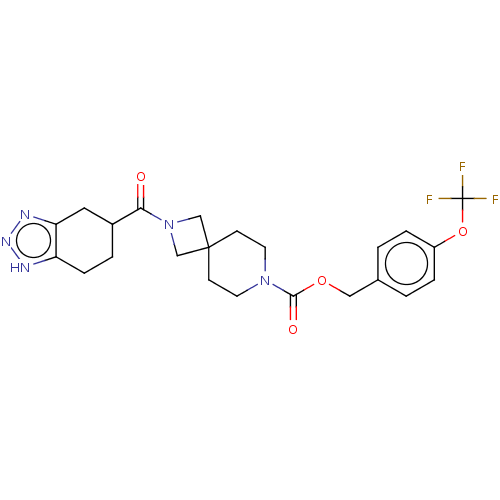 (US10633384, Example 12.43 | US9493486, 12.43) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM258585 (US10633384, 13.01 | US9493486, 13.01) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM440743 ((?)-2-Fluoro-4-(trifluoromethoxy)benzyl 2-(4,5,6,7...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10633384 (2020) BindingDB Entry DOI: 10.7270/Q2K077BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM442722 (US10647719, Example 3.00 | US10647719, Example 3.0...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM442722 (US10647719, Example 3.00 | US10647719, Example 3.0...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10647719 (2020) BindingDB Entry DOI: 10.7270/Q2V98C4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258501 (US10633384, Example 1.23 | US9493486, 1.23) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258574 (US10633384, Example 12.36 | US9493486, 12.36) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258575 (US10633384, Example 12.37 | US9493486, 12.37) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258580 (US10633384, Example 12.42 | US9493486, 12.42) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258581 (US10633384, Example 12.43 | US9493486, 12.43) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258585 (US10633384, 13.01 | US9493486, 13.01) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258590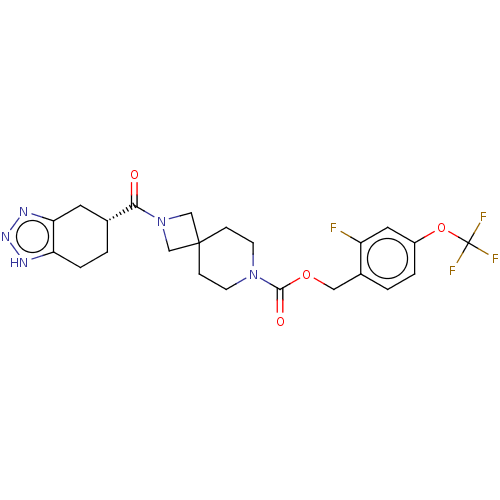 (US9493486, 15B) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556672 (US11352330, Example C5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556734 (US11352330, Example D50) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556539 (US11352330, Example A12) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556564 (US11352330, Example B2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556565 (US11352330, Example B3) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM477058 (US10882857, Example 2.06 | US11673888, Example 2.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NV9P52 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM477062 (US10882857, Example 4 | US11673888, Example 4 | [3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NV9P52 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM477064 (US10882857, Example 4.02 | US11673888, Example 4.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NV9P52 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511579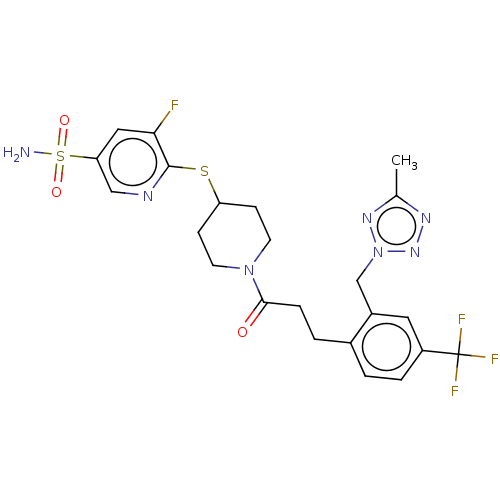 (US11059794, Example 6.39 | tert-butyl 4-(3-fluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556656 (US11352330, Example B88) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556661 (US11352330, Example B92) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556687 (US11352330, Example D5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556694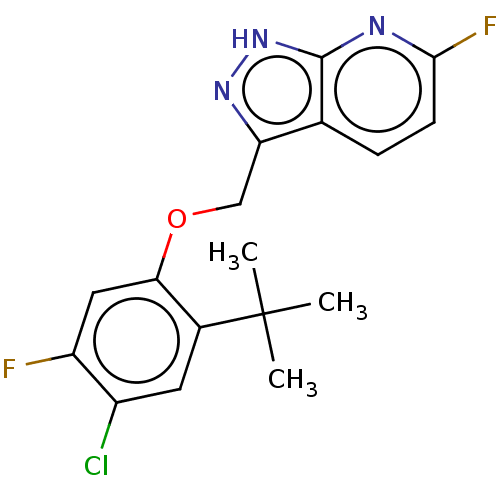 (US11352330, Example D12) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511567 (US11059794, Example 6.27 | tert-butyl 4-(2,3-diflu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511578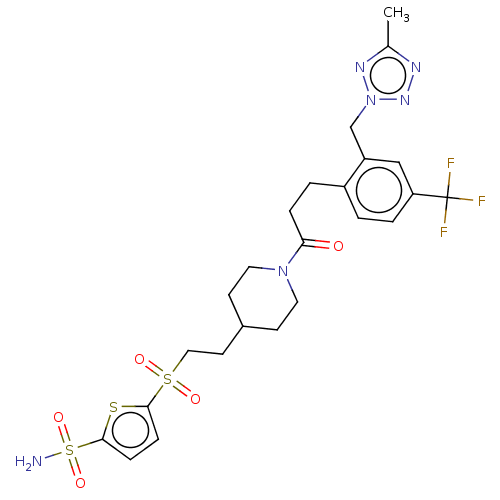 (US11059794, Example 6.38 | tert-butyl 4-[2-(5- sul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511538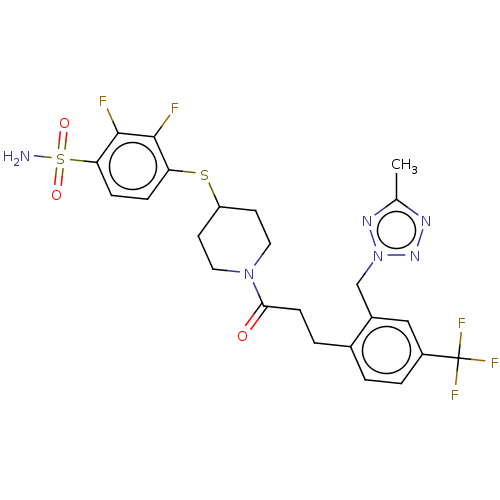 (US11059794, Example 6.00) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511541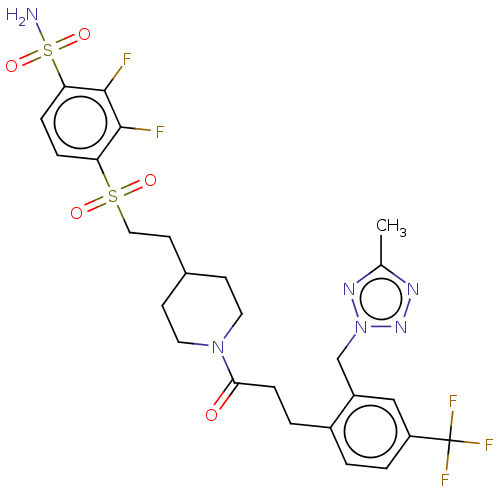 (US11059794, Example 6.03 | tert-butyl 4-[2-(2,3-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511551 (US11059794, Example 6.13 | tert-butyl 4-(2-fluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511558 (US11059794, Example 6.18 | tert-butyl 4-[2-(2-fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50187688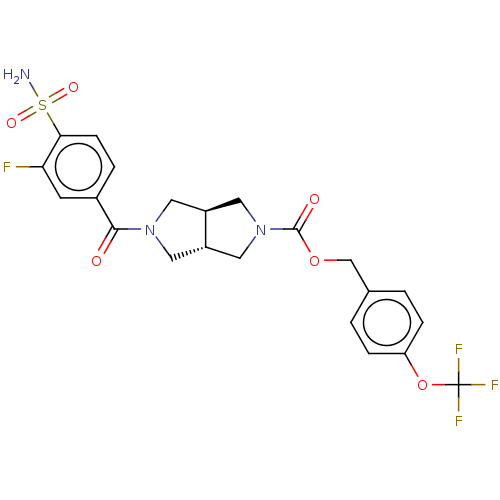 (CHEMBL3828650 | US10913745, Example 1.11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481899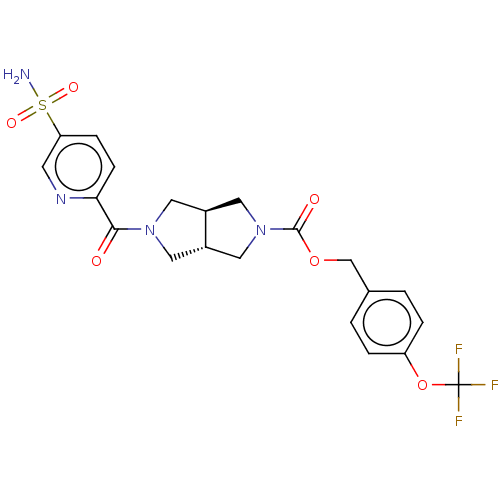 (US10913745, Example 1.13 | US10913745, Example 1.1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481899 (US10913745, Example 1.13 | US10913745, Example 1.1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481920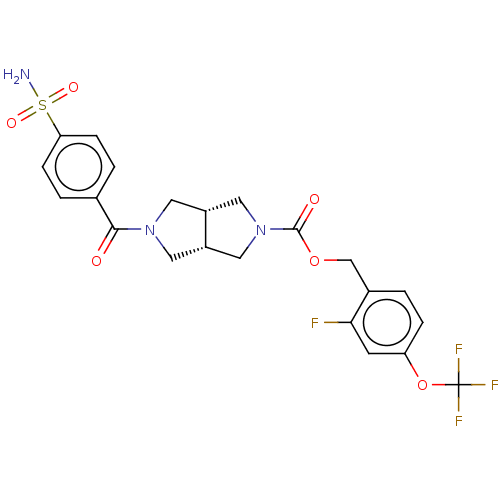 (US10913745, Example 7.01) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481930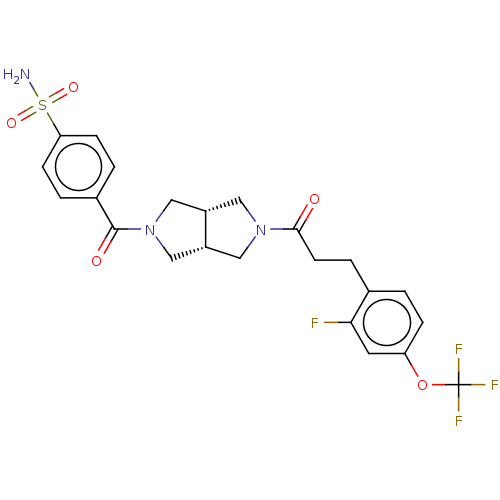 (US10913745, Example 8.07) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481932 (US10913745, Example 8.09) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511581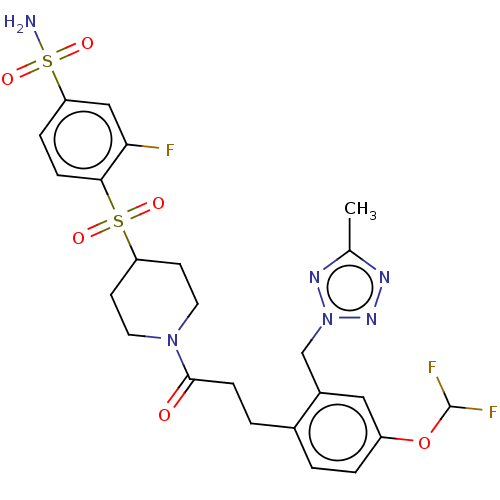 (US11059794, Example 6.41 | tert-butyl 4-(2-fluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511585 (US11059794, Example 6.45 | tert-butyl 4-[2-[(5-sul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511524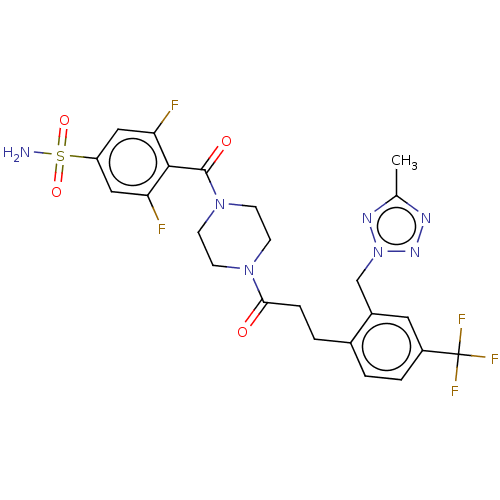 (US11059794, Example 4.00) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511530 (4-(piperidin-4- ylmethyl)benzenesulfonamide hydroc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM467326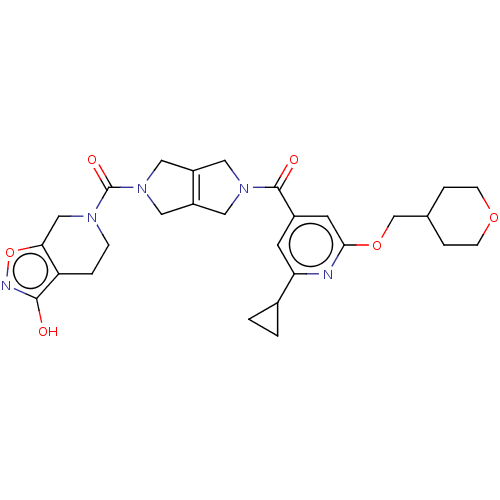 (US10800786, Example 2.01) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10800786 (2020) BindingDB Entry DOI: 10.7270/Q2RN3BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM467343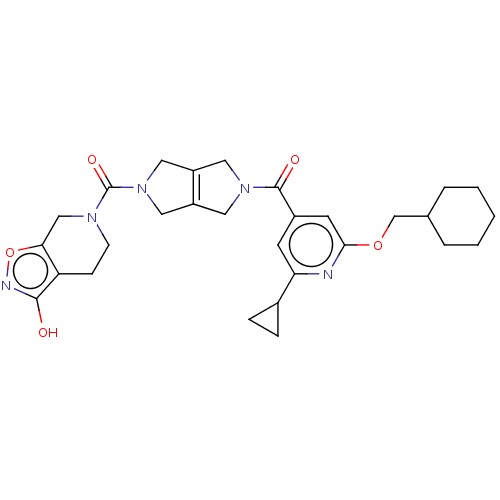 (US10800786, Example 2.15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10800786 (2020) BindingDB Entry DOI: 10.7270/Q2RN3BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM467346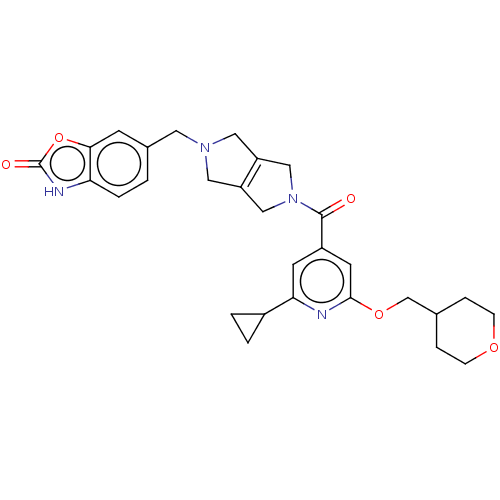 (US10800786, Example 5.00) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10800786 (2020) BindingDB Entry DOI: 10.7270/Q2RN3BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1968 total ) | Next | Last >> |