

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503111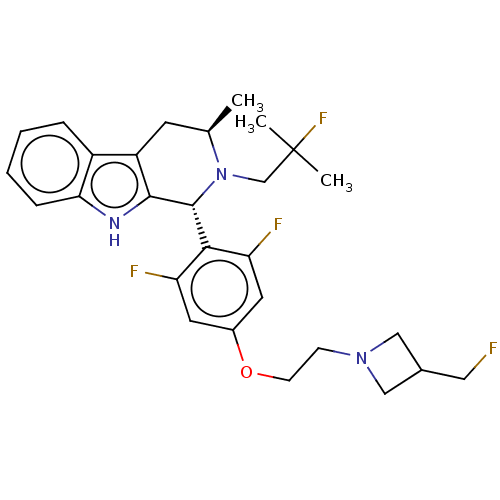 (CHEMBL4528514 | US11672785, Goodacre Compound 102 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells assessed as downregulation of ER alpha receptor expression measured after 18 to 22 hrs by immuno... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503109 (CHEMBL4591926) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells assessed as downregulation of ER alpha receptor expression measured after 18 to 22 hrs by immuno... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50125052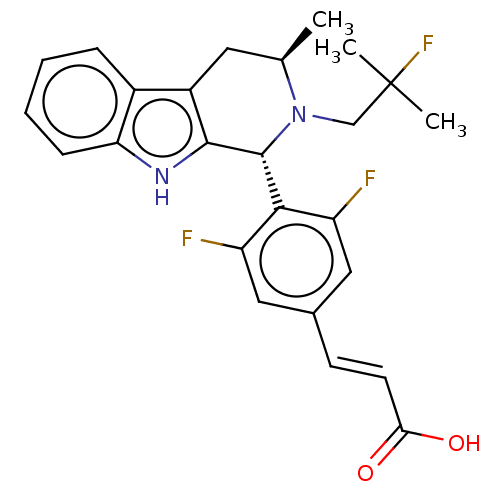 (CHEMBL3623004 | US10130617, Example 1 | US20240043...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells assessed as downregulation of ER alpha receptor expression measured after 18 to 22 hrs by immuno... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503114 (CHEMBL4447306) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluorescent labelled fluormone ES2 from human recombinant GST tagged ER alpha LBD ( 282 to 595 residues) expressed in baculovirus inf... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503112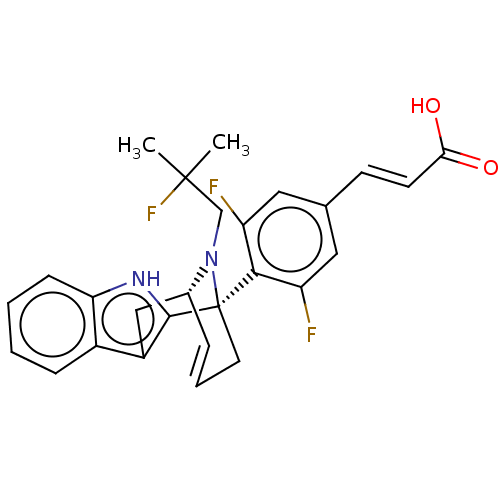 (CHEMBL4587197) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluorescent labelled fluormone ES2 from human recombinant GST tagged ER alpha LBD ( 282 to 595 residues) expressed in baculovirus inf... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503110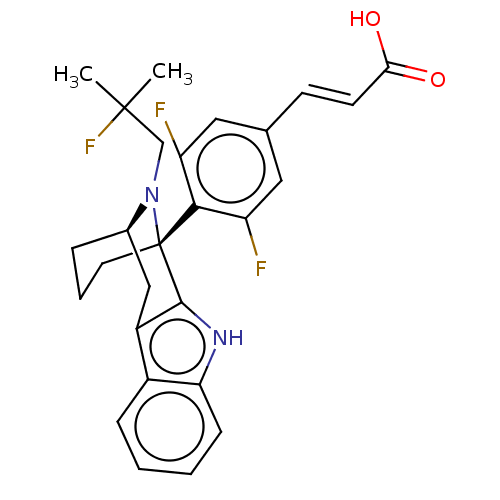 (CHEMBL4452972) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluorescent labelled fluormone ES2 from human recombinant GST tagged ER alpha LBD ( 282 to 595 residues) expressed in baculovirus inf... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50125052 (CHEMBL3623004 | US10130617, Example 1 | US20240043...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluorescent labelled fluormone ES2 from human recombinant GST tagged ER alpha LBD ( 282 to 595 residues) expressed in baculovirus inf... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503111 (CHEMBL4528514 | US11672785, Goodacre Compound 102 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluorescent labelled fluormone ES2 from human recombinant GST tagged ER alpha LBD ( 282 to 595 residues) expressed in baculovirus inf... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503109 (CHEMBL4591926) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluorescent labelled fluormone ES2 from human recombinant GST tagged ER alpha LBD ( 282 to 595 residues) expressed in baculovirus inf... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503114 (CHEMBL4447306) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells assessed as downregulation of ER alpha receptor expression measured after 18 to 22 hrs by immuno... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503112 (CHEMBL4587197) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells assessed as downregulation of ER alpha receptor expression measured after 18 to 22 hrs by immuno... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503113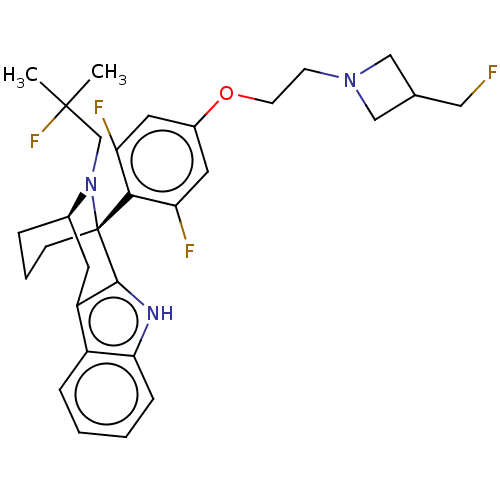 (CHEMBL4516412) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluorescent labelled fluormone ES2 from human recombinant GST tagged ER alpha LBD ( 282 to 595 residues) expressed in baculovirus inf... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503113 (CHEMBL4516412) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells assessed as downregulation of ER alpha receptor expression measured after 18 to 22 hrs by immuno... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503110 (CHEMBL4452972) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells assessed as downregulation of ER alpha receptor expression measured after 18 to 22 hrs by immuno... | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50450369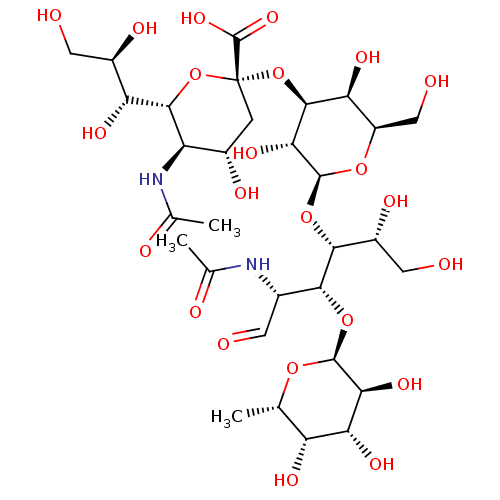 (SIALYL LEWIS X | Sialyl LeX | Sialyl lewis-x | sLe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Compound was evaluated for the Selectin E binding activity. | Bioorg Med Chem Lett 8: 2803-8 (1999) BindingDB Entry DOI: 10.7270/Q2RN38CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50503110 (CHEMBL4452972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50503110 (CHEMBL4452972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50503110 (CHEMBL4452972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50503110 (CHEMBL4452972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50503110 (CHEMBL4452972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 10: 1492-1497 (2019) Article DOI: 10.1021/acsmedchemlett.9b00370 BindingDB Entry DOI: 10.7270/Q21839SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||